Uses of hafnium and carbon compounds as the main components of ceramics. Hafnium carbide (HfC) theoretical density 12.7g/cm3, melting point
At 3890℃, it has the highest melting point of any known single compound. Volume resistivity 1.95&# 215; 10 - Ω & # 4
183; Cm (2900℃), thermal expansion coefficient 6.73&# 215; 10-6 / ℃. Usually hafnium dioxide (HfO2) is used with carbon in inert or
The powder is synthesized in reducing atmosphere, the reaction temperature is 1900 ~ 2300℃, and the high density is produced by hot pressing sintering or hot isostatic pressing
Ceramic dessert. Hafnium carbide can form solid solutions with many compounds (such as ZrC, TaC, etc.). For example, the group was divided into HFC-4TAC compound
Carbide, its microstructure is two phases: one phase is hafnium carbide - tantalum carbide eutectic; The other is a 50:1 appearance ratio
Acicular free graphite phase. This kind of... Near eutectic & # 8221; Hafnium carbide (HfC) products have good thermal stability and
High melting point, used as throat material for rocket nozzle. Very suitable for rocket nozzles, can be used as the nose of re-entry space rockets
Cone parts. Used in ceramics and other industries.
Constituent Properties
Properties: density 12.7g/cm3, melting point 3890℃, is known in the single compound of the highest melting point. Volume resistivity
1.95×10-4 ω •cm(2900℃), thermal expansion coefficient 6.73×10-6/℃. Usually hafnium dioxide (HfO2) is used with carbon in inert
Hafnium carbide can be synthesized with many compounds (such as ZrC, TaC) at reaction temperature 1900 ~ 2300℃
Etc.) to form a solid solution. With high melting point and high elastic coefficient, good electric conductivity, small thermal expansion and good impact performance.
Hafnium introduction
In nature, hafnium often symbiosis with zirconium, contain hafnium in the mineral that contains zirconium, hafnium and zirconium show kind qualitative coimage, hafnium basically occurrence is in
Zircon. Zircons for industrial use contain 0.5-2% HfO2. The beryllium zircon in secondary zirconium ores can be high in HfO2 content
Up to 15%. There is also a metamorphic zircon called kojite, which contains more than 5% HfO2. The latter two minerals are scarce in industry
Adopted. Hafnium is recovered mainly from the production of zirconium.
The smelting of hafnium and zirconium is basically the same, generally divided into five steps. The first step is the decomposition of ore. There are three methods:
(1) Chlorination of zircon to (Zr,Hf)Cl4.
(2) Alkali fusion of zircon, when zircon melts with NaOH at 600℃, more than 90% of (Zr,Hf)O2 is converted to
Na2(Zr,Hf)O3, in which SiO2 becomes Na2SiO3 and is dissolved in water. Na2(Zr,Hf)O3 dissolved with HNO3 can be prepared
Zirconium hafnium separation of the original solution, but because of containing SiO2 colloid, to the solvent extraction separation caused difficulties.
(3) Sintered with K2SiF6, K2(Zr,Hf)F6 solution was obtained after water immersion. Zirconium and hafnium can be separated from the solution by fractional crystallization.
The second step is the separation of zirconium and hafnium, using hydrochloric acid -MIBK (methyl isobutylketone) system and HNO3-TBP(tributyl phosphate) system
Conventional solvent extraction separation method. Using the difference in vapor pressure between HfCl4 and ZrCl4 melts at high pressure (above 20 atmospheres)
The technology of multistage fractionation has been studied for a long time, which can save the second chlorination process and reduce the cost. But since (Zr, Hf)Cl4 and HCl
The corrosion problem of ZrCl4 and HfCl4 is not easy to find the appropriate fractionation column material, but also will reduce the quality of ZrCl4 and HfCl4, increase the purification cost
To use. The third step is secondary chlorination of HfO2 to prepare crude HfCl4 for reduction.
The fourth step is purification and magnesium reduction of HfCl4. This process is the same as the purification and reduction of ZrCl4, and the semi-finished product is
Hafnium sponge.
The fifth step is vacuum distillation of hafnium sponge to remove MgCl2 and recover excess metal magnesium, and the finished product is sponge metal
Hafnium. If the reducing agent is sodium instead of magnesium, the fifth step is water immersion.
The hafnium sponge must be taken with great care from the crucible to avoid spontaneous combustion. Chunks of hafnium are broken up into smaller pieces of a certain size,
In order to press into a consumable electrode, and then cast into ingots. Spontaneous combustion should also be prevented when crushing. Further purification of hafnium sponge as with titanium and zirconium,
Thermal decomposition of iodide was used. The control conditions are slightly different from those of zirconium, the small pieces of hafnium sponge around the iodized tank are kept at a temperature of
600℃, and the center of the hot wire temperature of 1600℃, than the zirconium "crystal bar" 1300℃ for higher. Hafnium processing into
It includes steps such as forging, extrusion and tube drawing, the same method used to process zirconium.
The main use of hafnium is to make control rods for nuclear reactors. Pure hafnium has plasticity, easy processing, high temperature resistance and corrosion resistance,
It is an important material for atomic energy industry. Hafnium is an ideal neutron absorber with large thermal neutron capture cross section and can be used as atomic reactor
Control rods and protection devices. Hafnium powder can be used as propellers for rockets. Cathodes for X-ray tubes can be manufactured in the electrical industry. The hafnium
The alloy can be used for rocket nozzles and glider re-entry aircraft leading edge protection, hF-TA alloy for the manufacture of tool steel and
Resistance material. Hafnium is used as an added element in heat-resistant alloys, such as tungsten, molybdenum, tantalum, and some added hafnium in alloys. HfC because
High hardness and melting point, can be used as carbide additives. The melting point of 4TaC•HfC is about 4215℃, which is the highest known
Compounds.
Synthesis method
Usually with hafnium dioxide and carbon in inert or reducing atmosphere synthesis powder, reaction temperature 1900-2300℃, with hot pressing sintering method or hot isostatic pressing method to produce high density ceramic dessert.
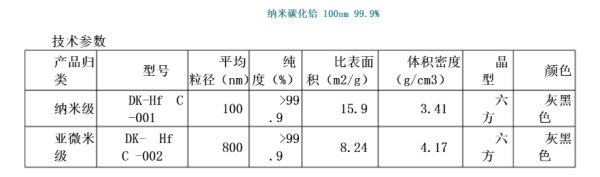
Main Features:
Superfine hafnium carbide powder is prepared by gas phase method of variable current laser ion beam. The powder has high activity and large surface energy. It is widely used in powder
At the end of metallurgy above, hafnium carbide (HfC)=185.501, containing carbon 6.47%, sodium chloride cubic crystal belongs to the crystallization of the system, theoretical density
12.7g/cm3, melting point 3890℃, is the highest in a single compound. Volume resistivity 1.95×10-4 ω •cm(2900℃),
Thermal expansion coefficient 6.73×10-6/℃. It has high elastic coefficient, good electric and thermal conductivity, small thermal expansion coefficient and good
Good impact resistance, superfine hafnium carbide can form a solid solution with many compounds (such as ZrC, TaC, etc.).
Application areas:
1 superfine hafnium carbide powder is suitable for rocket nozzle throat material field, is also an important cermet material;
2 Powder metallurgy;
3 High temperature resistant structure above.
Production method: sintering method, atomization method, solution method (carbon thermal reduction method)
(Hfc) =185.501, carbon content 6.47%, gray metal powder, belongs to sodium chloride cubic crystal system crystallization, theory
Density 12.7g/cm3, melting point 3890c, is the highest known single compound. Thermal expansion coefficient 6.73* 10-6C.
It has high elastic coefficient, good electrical and thermal conductivity, small thermal expansion coefficient and good impact resistance, very
Used in rocket nozzle material field, is also an important metal ceramic material.
The invention relates to a preparation method of one-dimensional hafnium carbide nanomaterial, which is characterized in that the preparation concentration is 0.05 ~
2mol/L Ni(NO3)2 ethanol or aqueous solution, put the clean graphite base in the solution to soak and dry; then
Suspended in the vertical tube resistance furnace for deposition, after deposition, stop feeding reaction gas, turn off the heating power naturally drop
One-dimensional HfC nanowire and nanoribbon were prepared. The method can be effectively controlled by taking advantage of the controllable CVD process
The morphology and size of one-dimensional HfC nanomaterials were studied to obtain HfC nanoribbons and HfC nanowire with high purity and performance. At the same time using
The deposition temperature is low, the deposition pressure is in the range of low vacuum, and the catalyst used is common catalyst
The cost of preparing high purity and high performance HfC nanowires is reduced.
A method for preparing one-dimensional hafnium carbide nanomaterial is characterized in the following steps: Step 1: the preparation concentration is 0.05 ~
2mol/L Ni(NO3)2 ethanol solution; Step 2: Soak the cleaned graphite base in the solution of Step 1
1h, make its surface attached with Ni(NO3)2, then take out the graphite base and put it in a drying oven at 40 ~ 55℃ to dry; Step 3:
The graphite substrate with Ni(NO3)2 on the surface in Step 2 was suspended in a vertical tubular resistance furnace and vacuumized to 2kPa, then
After that, inert argon was used as a protective gas to raise the furnace temperature to 1000-1200 ℃ at a heating rate of 5-10 ℃/min. step
Step 4: H2, HfCl4 and CH4 gases are introduced, and the partial pressures of H2, HfCl4 and CH4 are controlled to be 0.84 ~ 0.98 and 0.08 ~ respectively
0.01, 0.08 ~ 0.01, HfCl4 gas flow is 80 ~ 140mg/min; Step 5: Adjust the vacuum pump pumping speed to CVD
The deposition pressure in the furnace is controlled in 2kPa ~ 30kPa; The deposition time is 2-8h. Step 6: Stop backflow after deposition
One-dimensional HfC nanowire and nanoribbon were prepared by turning off the heating power and cooling naturally.
Hafnium dioxide (HfO₂) is a kind of ceramic material with wide gap and high dielectric constant, especially in the industry recently
The subfield is of great concern because it is the most likely to replace the metal oxide half-guide, the core component of current silicon-based integrated circuits
Volume field effect tube (MOSFET) gate insulation layer silicon dioxide (SiO₂) to solve the current MOSFET in the traditional SiO₂/Si junction
The dimensional limit problem for the development of structures.
Density: 9.68g/cm3 Melting point: 2850℃ Molar mass: 210.49 g/mol
CAS No. : 12055-23-1 Evaporation pressure: 1Pa at 2678℃; At 2875 ℃ for 10 pa
Linear expansion coefficient: 5.6×10-6/K Solubility: insoluble in water purity :99.99
Film properties: transmittance range ~ 220-12000nm; Refractive index (250nm) ~ 2.15 (500nm) ~ 2
Evaporation conditions: use electron gun, oxygen partial pressure ~ 1 ~ 2×10-2Pa. Evaporation temperature ~ 2600 ~ 2800℃, substrate temperature ~
250℃, evaporation rate 2nm/s
Application :UV anti-reflective film, interference film white powder, monoclinic, tetragonal and cubic crystal structure. Density, respectively,
Are 10.3, 10.1 and 10.43g/cm3.
Melting point 2780 ~ 2920K. The boiling point of 5400 k. Thermal expansion coefficient 5.8×10-6/℃. Insoluble in water, hydrochloric acid and nitric acid,
Soluble in concentrated sulfuric acid and hydrofluoric acid. From hafnium sulfate, hafnium chloride oxide compounds such as thermal decomposition or hydrolysis. For the production of metal hafnium and
Hafnium alloy raw material. Used as refractories, radiation-resistant coatings and catalysts.
The metal with the highest melting point at 1ATM is tungsten, which reaches 3410℃. Nonmetals are carbon (graphite, diamond)
The substance with the highest known melting point is the hafnium alloy (Ta4HfC5), up to 4215℃.20% HfC
And 80%TaC alloy has the highest melting point of any known substance at 4400℃ for a mixture of 4 parts tantalum carbide and 1 part hafnium carbide and its melting point
As high as 4215 ℃, it can be used as structural material on jet engines and missiles.
The melting points of metallic carbides, especially group ⅳ B, ⅴ B and ⅵ B, are all above 3273K, among which hafnium carbide,
Tantalum carbide, at 4160K and 4150K respectively, has the highest melting points of any known substance. Most carbides are very hard,
Their microhardness is greater than 1800kg•mm2
Microhardness 1800kg•mm2 is equivalent to mohs - diamond - 1 hardness 9). Many carbides do not decompose well at high temperatures,
The antioxidant capacity is stronger than its constituent metals. Titanium carbide has the best thermal stability of all carbides and is a very important metal
However, in an oxidizing atmosphere, all carbides are easily oxidized at high temperature, which may be said to be one of the carbides
Weaknesses:
Hot pressing sintering is a high temperature pressing forming diamond bit manufacturing method. The specific process is the skeleton metal and bonding metal
Are made into powder, according to a certain proportion in the ball mill fully mixed, in the tire material working layer mixed with a certain concentration of steel
Stone, layered into the stone mold; The steel body of the drill bit is placed in the material part of the tire body, and then heated at about 1000℃ for a certain time
Pressure (10 ~ 15 mpa) to form a diamond bit. Heating methods are resistance furnace, medium frequency induction furnace and other methods; add
The main use of pressure press or jack. The main advantage of hot pressing sintering for diamond drill is that the drill is formed in graphite mold
Type, so the shape is good, the size can meet the design requirements; Sintering temperature is low, sintering time is short, the quality of diamond itself
The quantity damage is small; No special protection gas, simple equipment; Short production cycle. The disadvantage is the poor continuity of batch production, drilling
Head quality is not easy to maintain stability.
--------------------------------------------------------
Author: Yang Yanshun
Link: https://wenku.baidu.com/view/3a590082a88271fe910ef12d2af90242a995ab6f.html
Source: Baidu Wenku
Technological innovation
Honesty is the foundation
Contact Number: +86-15698999555 |
Address: NO.6 ,SHENGHUA STREET,TAIHE DISTRICT, JINZHOU CITY, LIAONING PROVINCE, CHINA. |