Challenging the performance limits of materials or finding materials with extreme performance not only has important scientific value, but also has a strong demand for technological application background. In recent decades, the development of hypersonic aircraft has put forward increasingly stringent requirements for ultra-high temperature thermal protective materials, leading to a rapid increase in interest in ultra-high temperature materials. However, due to the extreme difficulty of precise experimental measurements under ultra-high temperature conditions, it remains an unsolved mystery to which material has the highest melting point.
Currently, a widely promoted viewpoint regarding materials with the highest melting point is that HfTa4C5 has the highest melting point of~4215 ° C among all known materials. This statement has been cited in many textbooks and recorded in the Encyclopedia Britannica. However, this viewpoint actually comes from a fallacy. In the original paper by Agte and Alterthum, the melting point of HfTa4C5 was reported as 4215 absolute temperature, which is 4215K, rather than 4215 ° C. In subsequent experimental studies, there has been controversy over whether HfTa4C5 has the highest melting point, with Andrievskii et al. suggesting that HfTa4C5 may have the highest melting point; The team led by Rudy believes that the melting point of the solid solution of hafnium carbide and tantalum carbide varies monotonically with the composition, and there is no intermediate component with the highest melting point. Although the topic of materials with the highest melting point has been discussed for nearly 100 years since Agte and Alterthum published their first research report in 1930, and new experimental techniques are constantly being used for related testing, there are still many difficulties in hoping to completely solve this challenge through experiments.
Recently, research teams from Beijing Institute of Science and Intelligence, Harbin Institute of Technology, Institute of Aerospace Materials and Processes, and Beijing Shenshi Technology Co., Ltd. conducted in-depth theoretical research on the highest melting point material by combining deep potential molecular dynamics simulation and Bayesian global optimization. They revealed the relationship between the melting point of Hf Ta-C-N system materials and their composition, and predicted the potential composition of the highest melting point material.
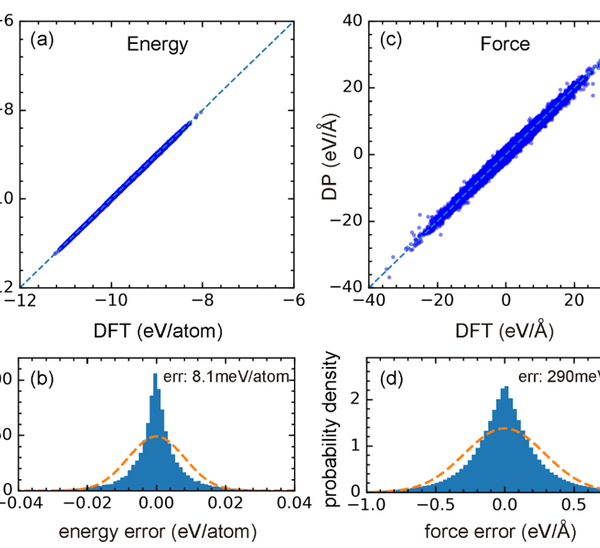
In order to overcome the problem of insufficient accuracy in traditional molecular dynamics prediction, the paper uses first-principles calculation data and deep potential energy method to train the potential function model of the Hf Ta C-N system. The trained deep potential energy model can achieve computational accuracy close to first principles, with predicted energy and atomic force errors of 8.1 meV/atom and 290 meV/Å relative to first principles, respectively. In addition, the accuracy of the deep potential energy model was further verified through properties such as equilibrium lattice constant, elastic modulus, and equation of state.
Picture 1_ Copy. png
Figure 1. (a) and (c): Comparison of energy and atomic forces predicted by DP (Deep Potential Energy) with DFT (Density Functional Theory); (b) The error distribution of energy and atomic force prediction of DP relative to DFT.
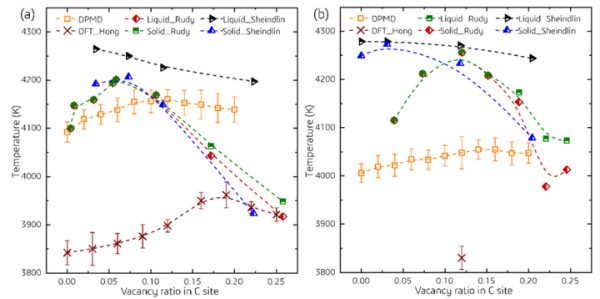
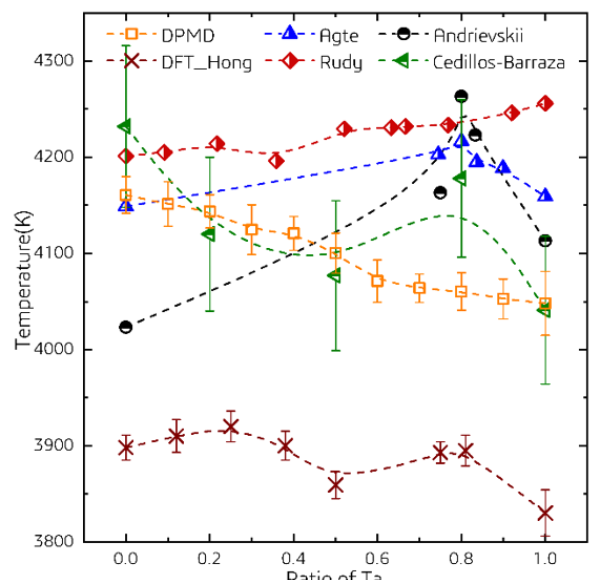
Based on this high-precision machine learning potential function model, the article first calculates the melting points of HfC1-x, TaC1-x, and Hf1-yTayC1-x as a function of their composition. The calculated predicted melting point is close to the experimental test results, verifying the reliability of this method in predicting melting point. The calculation results indicate that: (1) For HfC and TaC, the introduction of C vacancies can indeed significantly improve the melting point of the material. When the concentration of C vacancies is between x and 12%, the melting points of HfC1-x and TaC1-x reach their maximum values. (2) For Hf1-yTayC0.88 solid solution, the calculation results show that its melting point monotonically changes with Ta content,
There is no maximum melting point observed in a certain component. This conclusion is consistent with the conclusion of Rudy et al. and does not support the assertion that HfTa4C5 has the highest melting point.
Picture 2_ Copy. png
Figure 2. The variation of melting point with carbon vacancy concentration calculated by DPMD and its comparison with DFT and experimental results (a) HfC1-x, (b) TaC1-x.
Picture 3_ Copy. png
Figure 3. The relationship between the melting point of Hf1-yTayC0.88 calculated by DPMD and the variation of Ta content, as well as its comparison with DFT and experimental results.
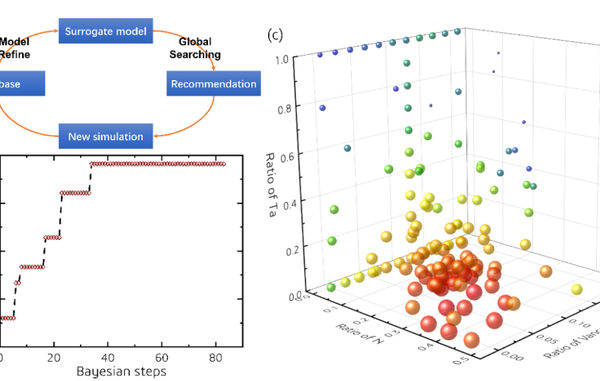
In order to further explore which component has the highest melting point, researchers subsequently conducted extensive searches for the melting point of the Hf Ta C-N system (Hf1-yTayC1 x-zNz) using Bayesian global optimization algorithm and DPMD. Thanks to the efficiency of the Bayesian algorithm, the new component with the highest melting point, HfC0.638N0.271 (melting point~4236K), was obtained in less than 50 steps of search, which is about 70K higher than the highest value in the Hf-C binary system (HfC0.88, melting point~4167K). It can be seen that compared to the C vacancy, solid solution N will be a more effective means to improve the melting point of HfC. The main mechanism by which N addition enhances the melting point of HfC is believed to be the instability of C-N and N-N bonds in the liquid phase, which reduces the liquid phase entropy and leads to a decrease in the stability of the liquid phase relative to the solid phase.
Picture 4_ Copy. png
Figure 4. (a) Schematic diagram of searching for the highest melting point material based on Bayesian optimization, (b) Changes in the highest melting point value with iteration steps during the search process, and (c) Changes in the melting point of the Hf-Ta-C-N system (Hf1-yTayC1-x-zNz) with composition (the larger the ball, the higher the melting point).
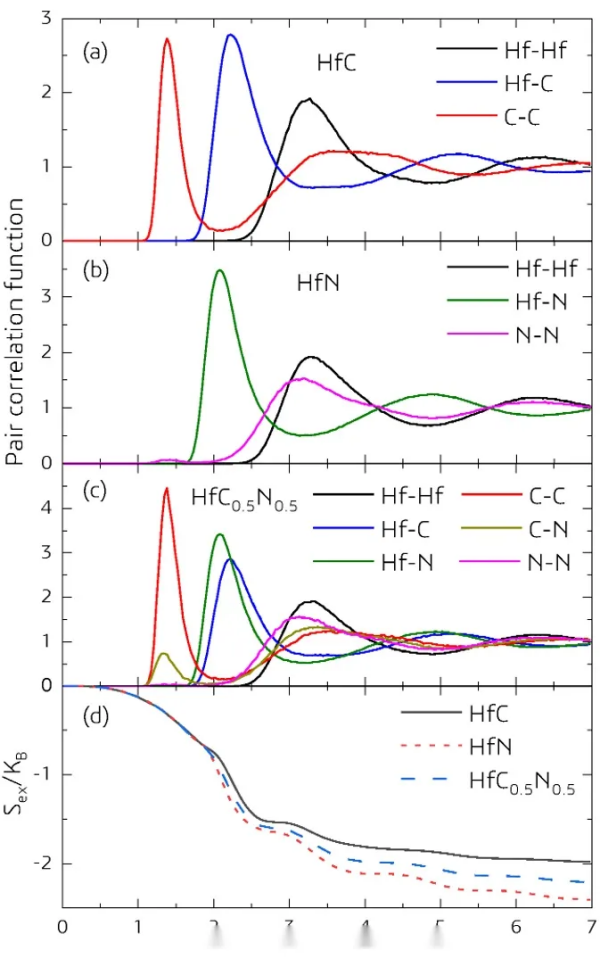
Picture 5_ Copy. png
Figure 5. Correlation functions (normalized to r → ∞) for (a) HfC, (b) HfN, and (c) HfC0.5N0.5 liquid phases. (d) Comparison of excess entropy of ideal gases in liquid phase HfC, HfN, and HfC0.5N0.5. Compared to the formation of obvious C-C bonds in HfC, the N-N and C-N bonds in HfN and HfC0.5N0.5 liquid phases are much less, indicating their instability.
Overall, this article reveals the relationship between the melting point of Hf Ta C-N system and its composition, as well as its underlying mechanism: (1) C vacancies can effectively enhance the melting points of HfC and TaC; (2) The solid solution melting points of HfC and TaC are between the two, and higher melting points cannot be obtained; (3) The addition of N can more effectively improve the melting point of HfC compared to the vacancy of C. The relevant research provides new ideas for the composition design of ultra-high temperature materials and also provides new directions for the selection of ultra-high temperature thermal protection materials.
Technological innovation
Honesty is the foundation
Contact Number: +86-15698999555 |
Address: NO.6 ,SHENGHUA STREET,TAIHE DISTRICT, JINZHOU CITY, LIAONING PROVINCE, CHINA. |