PRINCIPLE AND TECHNICAL PROCESS OF
PRODUCING SILICOCALCIUM
Xu Luming
(Capital Steel Mill Ferroalloy Branch, Beijing 100023, China)
Abstract It discusses the physicochemical principle of producing silicocaleium and introduces four kinds of domestic and foreign techniques, such as, mixing process, two-step
process,slicingprocessandelectro silicothermic process. The disadvantages and advantages of the above mentioned techniques are all explained. It puts forwards the principal contradictions in producing silicocalcium and as well as its improvement measures. Slicing process is a kind of technique in producing silicocalcium through domestic exploration. It points out that large scale furnaces and the application of slicing process is the fundamental way for silicocalcium production.
Keywords silicocalcium, technical process, principle,equipment
Silicon calcium alloy is an ideal Oxygen scavenger and desulfurizer for smelting high-quality steel, and it is a calcium treated alloy that must be used for continuous casting steel, especially for continuous casting of aluminum containing steel, in order to prevent casting mouth from bulging (looping). Adding silicon calcium alloy to the steel during the steelmaking process can change the behavior of residual inclusions in the steel, reduce the content of inclusions in the steel, and improve the mechanical properties of the steel. It is a purifying agent used in the production of high-purity steel. In the past twenty years, there have been significant improvements in the method and process of adding silicon-calcium alloy to molten steel. The process of injecting bulk alloy into the ladle and powder injection (spray metallurgy) into the ladle has now been improved to use silicon-calcium powder cored wire to feed into the ladle or LF refining furnace through a wire feeder. The adoption of wire feeding technology greatly improves the utilization rate of calcium element in molten steel.
The silicon calcium alloy smelting process was successfully developed by French BOZEL company in the early 20th century. It is divided into four methods: two-step method, mixed feeding method, layered feeding method (layered method), and electrosilicothermal method. The layered feeding smelting process was successfully studied by the original Beijing Ferroalloy Factory in 1964. The outstanding advantages of the new process are low energy consumption, long furnace service cycle, and high product quality. Up to now, the production of silicon calcium alloy using the layered method new process still accounts for more than 50% of the total production in China, and its operation is difficult. So far, it can only be carried out on electric furnaces with transformer capacity equal to or less than 1.5MVA. Electric furnaces with a capacity of 1.5MVA or above in China are all produced using a hybrid method, and their transformer capacity is generally below 3.6MVA. Compared with advanced industrial countries, there is a significant gap in the production process, product quality, energy consumption, environmental protection, and electric furnace capacity indicators of silicon calcium in China, especially in the capacity of electric furnaces. There is no production of silicon calcium electric furnaces above 6MVA in China, and the small capacity of electric furnaces is one of the key factors restricting the development of silicon calcium products in China. The smelting power consumption of large silicon calcium electric furnaces with a capacity of 10MVA and above in foreign countries is maintained at a level of 10000kWh/t, and the furnace body has a lifespan of over 1 year. At present, Shaanxi Shenghua Smelting and Chemical Company is building a large 30MVA silicon calcium electric furnace, which will be the largest capacity silicon calcium electric furnace in China after completion and operation.
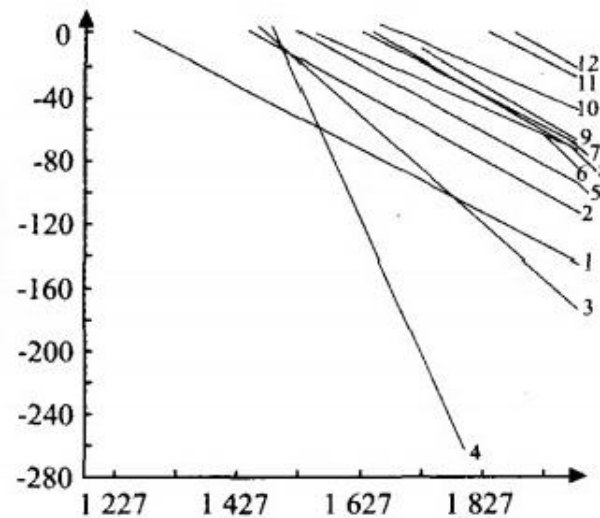
Fig.1.Relations between temperature and free energy of silico- calcium which Si and Ca reduced by carbon
From Figure 1, it can be seen that the standard free energy change of the reduction reaction in the calcium silicate furnace increases with the negative value of △ G as the temperature increases. The larger the negative value of △ G, the stronger the chemical reaction is, and it proceeds at a faster reaction rate to the right. Based on this, the main reactions in the silicon calcium alloy furnace and possible situations will be analyzed and discussed.
From Figure 1, it can be seen that during the smelting process, under low furnace temperature conditions, a chemical reaction equation (1) is first generated, which is carried out under conditions of high carbon content and low furnace temperature. This indicates that the generation of SiC in the furnace is inevitable in the process of producing calcium silicate using the mixed method. From Figure 1 or reactions (12) and (14), it can be seen that the decomposition and reduction reaction of SiC can only be carried out at high temperatures above 1875 ℃ or 2115 ℃, indicating that it is not easy to decompose and destroy SiC with CaO and SiO ₂ after its formation. The melting point of SiC is 2540 ℃, and it often crystallizes preferentially in the slag compared to other components. Most SiC will accumulate at the bottom of the furnace. In the process operation, efforts should be made to reduce the generation of SiC in the furnace, so that the furnace material can quickly enter the high-temperature area, maintain the furnace temperature, and control the progress of reaction equation (1).
From Figure 1 and reaction equations (2) and (7), it can be seen that the reaction of using CaC ₂ to reduce SiO ₂ to generate CaSi and the negative rate of free energy change increases rapidly with the increase of temperature, indicating that the reactions in furnace (2) and (7) are easier to carry out. It is feasible to first use CaO to generate CaC ₂ in the furnace or use additional CaC ₂ as a reducing agent to produce silicon calcium alloy.
From Figure 1 and reaction equation (3), it can be seen that the SiO ₂ in the furnace is partially reduced to SiO, and the negative free energy value increases quickly, making the SiO generation reaction easy to proceed. However, SiO produced is prone to volatilization and loss, so it is necessary to maintain a certain thickness of material layer in production to absorb gaseous SiO and transform it into Si or SiC, thereby improving the recovery rate of Si element.
From reaction equation (4) and Figure 1, it can be seen that in the presence of iron, the reaction of reducing CaO and SiO ₂ with carbon can start at a lower temperature. The slope of line (4) is the highest, and the chemical reaction will proceed at a faster rate to the right, indicating that the presence of iron is beneficial for the production of silicon-calcium alloys. It is the theoretical basis for producing silicon calcium alloys and low-grade silicon calcium alloys.
Reaction (5) indicates that in the presence of carbon, the slope of the free energy change of the reaction of SiO ₂ and CaC ₂ producing CaSi ₂ is greater than that of reaction (2) and other free energy change lines producing CaSi ₂ and CaSi alloys, indicating that the negative value of Δ G Å ° increases faster with temperature. Under certain conditions, this reaction will play a major role in the production of silicon calcium alloys.
Reaction (6) is a reaction in which SiO ₂ is reduced by carbon alone to form Si. It operates at higher temperatures.
Reactions (8) and (10) indicate that when CaO and SiO ₂ are reduced with carbon to smelt silicon-calcium alloys, SiC and Ca elements in the vapor state will be generated. Their theoretical starting reaction temperature is slightly lower than the temperature required to generate CaSi ₂. This theory is applicable to the process of producing silicon-calcium alloys without the participation of CaC ₂ in the reaction. When there is excessive carbon in the furnace charge, reaction (8) is easy to proceed, and the consequence is a large amount of volatilization loss of calcium vapor. When the temperature is above 1702 ℃, it is possible to directly reduce CaO and SiO ₂ by carbon to produce CaSi alloy without the use of the intermediate product CaC ₂ in the production of silicon calcium alloy. This is the basic physicochemical reaction and theoretical basis for the production of silicon-calcium alloys using the mixed feeding method. The temperature required for this chemical reaction is higher than that of the layered feeding method using CaC ₂ as an intermediate.
Reactions (11), (12), (13), and (14) are all reactions in which SiC is destroyed in the furnace. These reactions can only be carried out at high temperatures exceeding 1850 ℃ or 2200 ℃, indicating that the decomposition and destruction of SiC is difficult.
Reaction (15) indicates that it is extremely difficult to directly reduce CaO with carbon to generate calcium silicate. Reaction (16) indicates that CaC ₂ is less easily destroyed by CaO.Phase diagram analysis of 2 silicon calcium alloys
The temperature and composition relationship of the new silicon calcium alloy phase diagram is shown in Figure 2.From the phase diagram, it can be seen that Si and Ca can form three types of silicon calcium compounds, namely Ca ₂ Si, CaSi, and CaSi ₂, with melting points of 1314 ℃, 1224 ℃, and 1040 ℃ (decomposition temperature), respectively. From the thermodynamic data analysis of physicochemical reactions, it can be concluded that CaSi ₂ has the highest negative reaction free energy and stability. This indicates that its calcium vapor pressure is low and the evaporation loss during the production process is small. If viewed from the physical properties of silicon calcium, its melting point is low, which is beneficial for product separation. From the composition analysis of the product standards for producing silicon calcium alloys (Ca28% -31%, Si55% -65%), its melting point is about 1030 ℃, which is beneficial for product fluidity. Comparison of Production Methods and Process Characteristics of 3 Silicon Calcium Alloys
The industrial production of silicon calcium alloy currently adopts four process methods at home and abroad: mixing method (one-step method), two-step method, layering method, and electrosilicothermal method.

Fig.2 New phase diagram of silicocalcium
3.1 Mixing method
Also known as the "one-step method", it is the process of loading well weighed and evenly mixed dry lime, silica, and carbon reducing agent into a submerged arc furnace, and using processes such as ramming, ventilation, and calcination to produce silicon calcium alloys containing 55% to 60% Si and 28% to 31% Ca.
The main chemical reaction of this method is equation (10), which is multiplied by 5 to obtain the following equation:
CaO()+2SiO ₂)+5C (g)=CaSi ₂ ()+5CO (g)
△ G θ= 2039972-1032.8 T J/mol · CO T on=1702 ℃
From the perspective of Chemical thermodynamics, it is obvious that the above reaction must exist, but the reaction must be carried out at high temperature.
In actual production, the situation is more complex due to the addition of CaO into the furnace
SiO ₂ is a mixture that is prone to the following slagging reactions:
2CaO+SiO ₂=SiO ₂ · 2CaO
△ G θ=- 144348-13.97 T
A large amount of low melting point slag is formed, and this silicate can only be decomposed and reduced at high temperatures above 2300 ℃. The slagging reaction reduces the activity of CaO and SiO ₂ in equation (10), while the formation of low melting point slag reduces the temperature of the reaction zone, making the reaction of generating CaSi ₂ (10) more difficult to carry out.
To increase the furnace temperature, a method of increasing the slag melting point is adopted, which involves adding excess carbon to the furnace material to promote reactions (1) and (9).
SiO ₂ (s)+3C (g)=SiC (s)+2CO (g) T open=1235 ℃ CaO (s)+3C (m)=CaC ₂+CO (g) T open=1864 ℃
(1) The high melting point components SiC (melting point 2540 ℃) and CaC ₂ (melting point 2300 ℃) generated by the reaction enter the slag, increasing the slag melting point and slag formation temperature to obtain the high temperature required for the reaction (10) to proceed.
Due to the generation and deposition of SiC and CaC ₂ at the furnace bottom, the furnace bottom rises, which requires the use of reactions (2) and (12), namely:
CaC ₂+SiO ₂=CaSi+2COT T on=1433 ℃
2SiC+SiO ₂=3Si+2CO1 T open=2076 ℃
To destroy the carbides generated in the furnace to control the rise of the furnace bottom and generate the required silicon calcium alloy. This needs to be achieved by regularly adding a portion of silica separately to the furnace. The above are the principles and process methods based on which the hybrid method is based, with the following characteristics:
(1) The process operation is simple and the furnace condition is easy to grasp.
(2) Due to the slag formation reaction of calcium silicate, the slag amount increases and the recovery rate of Ca and Si elements is low.
(3) The addition of excessive carbon reduces the specific resistance of the furnace material, makes it difficult for electrodes to be inserted downwards, loosens the material layer at the furnace mouth, causes large heat loss in the furnace, and there are many phenomena of material collapse and fire escape at the furnace mouth.
(4) Due to the fact that SiC is easier to generate than CaC ₂ and is difficult to break down, the slag contains more SiC. This type of slag has a higher density than the alloy and is located in the lower part of the alloy. The melting point of SiC in the slag is very high, which crystallizes before other components in the slag and deposits at the bottom of the furnace, forming furnace nodules with SiC skeleton, causing the furnace bottom to continuously rise and shortening the production cycle. The furnace cycle of the mixed method is generally 3 months in foreign countries, and the cycle of the rotating furnace body can reach more than 6 months. When the mixed method process is used for production in China, the furnace service cycle is generally 20-30 days.
3.2 Two step method
The two-step process involves first mixing high-quality lime with CaO ≥ 80% and a carbon reducing agent according to reaction formula (9)
CaO (.)+3C (q)=CaC ₂ (s)+CO (g)
To produce calcium carbide, CaC2 is first refined in an electric furnace, and then the produced CaC ₂ is cooled and crushed. Then, a corresponding amount of silica and carbon reducing agent is added to produce silicon calcium alloy in another electric furnace. The main chemical reaction is as follows: CaC ₂ (s)+2SiO ₂ ()+2C (g)=CaSizc)+4CO (g). This process avoids the contact between CaO and SiO ₂ in the feeding operation, solving the problems of low slag forming temperature and difficulty in raising furnace temperature. This method does not require the addition of excessive carbon reducing agents, and basically avoids the accumulation of carbides in the furnace and the rise of the furnace bottom, enabling continuous production of the furnace without periodic shutdown. However, this process has the following drawbacks:
(1) Two electric furnaces and related equipment are required (one furnace for producing calcium carbide and one furnace for producing silicon calcium alloy).
(2) High comprehensive power consumption and unreasonable utilization of thermal energy. As the raw material of the Stöð 2 electric furnace, calcium carbide needs to be cooled, remelted and heated, which consumes a lot of heat energy. Moreover, the calcium carbide crushing process is prone to water absorption and pulverization, resulting in significant mechanical crushing losses.
(3) Strict requirements for moisture in raw materials. Since carbide is easy to absorb water and decompose to produce acetylene gas (CaC ₂+2H ₂ O=Ca (OH) ₂+C ₂ H ₂ T), the added carbonaceous reducing agent needs to be baked and dried before being put into the furnace. In addition, the utilization rate of raw materials is low, and a large amount of Ca (OH) ₂ generated after decomposition is also introduced, resulting in poor air permeability of the electric furnace. CaC ₂ decomposed by moisture can cause explosive and unsafe discharge of C ₂ H ₂, leading to deterioration of working conditions.
3.3 Layered feeding method
By comprehensively comparing the advantages and disadvantages of the mixed method and two-step method, and based on the principles of silicon calcium alloy production, the original Beijing Ferroalloy Factory gradually explored and created a new silicon calcium alloy production process in production practice, namely the layered feeding method (layered method).
The basic principle of the layering method is to change the feeding process in the same submerged arc furnace, so that the two-step smelting process can be achieved in one electric furnace. From the perspective of process operation, it is possible to reduce the contact between CaO and SiO ₂ to generate low melting point calcium silicate slag, thus eliminating the need for excessive carbon operation to reduce the formation of SiC, reduce the phenomenon of carbide deposition in the slag at the bottom of the furnace, and extend the smelting cycle of the electric furnace. The measure is to first add lime and carbon reducing agent in the same furnace to generate CaC ₂, and then add SiO ₂ (silica) to the furnace in a hot state to destroy CaC ₂. Simply put, it is to first smelt low gas producing calcium carbide in the furnace, without the liquid calcium carbide coming out of the furnace, and then add silica to the liquid calcium carbide to generate silicon calcium alloy. This new process greatly improves the utilization rate of electricity and reduces the unit power consumption of finished products.
The original Beijing Ferroalloy Factory began refining silicon-calcium alloy using a new layered process on a 0.4MVA electric furnace in 1964, achieving good results in improving element recovery rate and reducing power consumption. For forty years, the factory has been using the layering method for production. In March 1982, this new process was applied to a 1MVA electric furnace and achieved good results. The production indicators of silicon calcium alloy for this factory and a domestic factory are listed in Table 1.
The characteristic of the layering method operation process is that each furnace is divided into three stages of smelting: the first stage is the heating and dry burning of the furnace bottom after tapping, during which no new materials are basically added to the furnace, and the time is about 1/6 and 1/8 of the entire furnace smelting time; The second stage is the formation of calcium carbide (CaC ₂), in which all the lime and carbon reducing agent required for smelting low-grade calcium carbide are poured into the high-temperature area around the electrode in one go for calcium carbide smelting.
In recent years, there have been reports in foreign literature on the production of silicon-calcium alloys using the approximate layering method. West Germany reported that the former Soviet Union successfully first generated CaC ₂ from CaO in a large electric furnace, and then added quartz and other carbon to form alloys. Reference [5] also introduces a method for smelting calcium silicate slag with the highest possible amount of dissolved CaC ₂. First, the lime and carbon required to generate CaC ₂ are added, and then CaC ₂ and SiO ₂ are reacted to form an alloy until the CaC ₂ in the slag decreases. Then, lime and carbon are added again to generate CaC ₂. This is consistent with the theoretical basis of layered feeding pioneered by the original Beijing Ferroalloy Factory as early as 1964. The author believes that the "layered feeding method", a new process for smelting silicon calcium alloy, should continue to be studied and improved, and strive to promote its application in large electric furnaces, which will definitely achieve greater economic benefits.
3.4 Electrosilicothermal method
4CaO+6Si (micro ionization)+Fe ₂ Si=2CaSi ₂ Fe ₂ Si+2CaO · SiO ₂
Due to the presence of a certain amount of iron in the alloy, the reaction is easy to proceed, avoiding the generation of any carbides that may cause the furnace bottom to rise during production. The production efficiency is high, and it can achieve periodic production. However, due to the presence of a certain amount of iron in the raw material, only low-grade silicon calcium alloys can be produced.
The main contradiction in the production of silicon calcium alloy - furnace bottom rise and periodic shutdown
During the production process of calcium silicate, the furnace bottom rises and is forced to periodically shut down, which limits the improvement of productivity and significantly increases the unit power consumption of the product, reducing economic benefits. Therefore, it is a key issue that domestic silicon calcium production enterprises urgently need to study and solve. For many years, domestic enterprises have been producing silicon calcium alloys in small electric furnaces. The production cycle of the layering method is usually 10-12 months, usually 7-8 months, and the production cycle of the mixed method is generally about 1 month.
In order to extend the production cycle of the silicon calcium alloy electric furnace and control the rise of the furnace bottom, the original Beijing Ferroalloy Factory conducted a hot section of the silicon calcium electric furnace during the production process, observed the rising furnace bottom and furnace lump, and sampled and analyzed their chemical composition and petrographic structure. The results are shown in Table 2.
Table 2 Chemical composition of furnace bottom and furnace lump%
Tab. 2 Chemical composition of furniture retention and bottom%
Enterprise WeChat screenshot_ 1687309441227_ Copy. png
Petrographic analysis of furnace bottom nodules:
(1) The main mineral is SiC, evenly distributed and dense, with a volume content of 60% to 65%.
(2) Crystals form both idiomorphic and semi idiomorphic crystals.
(3) Other minerals include β CaO · SiO ₂, with relatively fine particles, filled in SiC gaps, with a content of about 5% to 10%; The content of CaC ₂ ranges from 1% to 5%.
The reason for the formation of furnace bottom nodules is due to the presence of reactions (1) and (9) in the furnace during the production process. When there is a large amount or uneven distribution of carbon reducing agents, it causes local high carbon content in the reaction zone inside the furnace. When the furnace temperature is not high, reactions (1) and (9) are promoted, producing a large amount of SiC (melting point 2500 ℃) and CaC ₂ (melting point 2300 ℃). These high melting point components exist in the slag. When the furnace temperature decreases, SiC and CaC ₂ crystallize preferentially over other components of the slag. In addition, due to the fact that the specific gravity of the slag in the production of silicon calcium alloy is greater than the specific gravity of the alloy, SiC first precipitates at the bottom of the furnace as a skeleton, and CaC ₂ combines with each other as fillers to form high melting point furnace nodules, causing the furnace bottom to continuously rise, forcing the electrode to lift up, and the melting zone inside the furnace to continuously move up, resulting in deterioration of the furnace condition, Finally, the furnace was forced to shut down. After the furnace is shut down, it is necessary to first cool the electric furnace (usually by spraying water into the furnace) and remove all condensed furnace materials and molten materials. Then, repair the furnace lining and tapping hole, and re dry the furnace to feed for a new furnace smelting. This process usually takes 2-3 days, not only consuming a large amount of materials, but also dissipating all the accumulated heat energy in the furnace. To extend the production cycle of the electric furnace, the following measures have been taken: (1) The layered method is used for the production of silicon calcium alloy. In terms of process operation, measures such as dry burning the furnace bottom after discharging, early temperature raising, and braising are adopted to promote the increase of furnace temperature and smooth the Chemical decomposition of CaC ₂ and SiC. With proper operation, the smelting cycle can be as long as 10-12 months. (2) Strict operation, selecting high-quality carbon reducing agents with higher resistance, appropriate dosage (usually theoretical carbon dosage), uniform mixing, ensuring deep electrode insertion and normal furnace conditions during the smelting process. When using the mixed method for smelting, the carbon content in the furnace charge is 1.1-1.2 times the theoretical carbon content, with the aim of generating a certain amount of SiC and CaC in the slag to improve the melting point of the slag. Then, a partial addition of SiO ₂ (silica) is used to destroy the CaC ₂ in the slag, in order to slow down the rise of the furnace bottom. (3) After casting, do not add new furnace materials and immediately power on. Contact the arc with the carbide on the furnace bottom, dry burn the furnace bottom, and use high temperature to promote the reaction of destroying the carbide, slowing down the rise of the furnace bottom.
(4) Adding iron material to the furnace and converting it to low-grade silicon-calcium alloy to destroy the furnace bottom nodules mainly composed of SiC.
In order to explore the law of conversion, in May 1978, the original Beijing Iron and Steel Institute and the original Beijing Ferroalloy Factory conducted an experiment to convert 45% ferrosilicon from silicon-calcium alloy, and then convert 45% ferrosilicon to ferrosilicon alloy.
The test was conducted on an electric furnace with a capacity of 0.4 MVA. The experimental results show that adopting the method of converting 45% ferrosilicon is completely possible to reduce the furnace bottom, eliminate the periodic production of silicon calcium alloy, and save electricity and reduce heavy cleaning labor. However, due to some issues with the sales of 45% ferrosilicon and the supply of scrap steel, this measure was not promoted at that time.
Energy saving and consumption reducing measures for the mixed process of 5 3.6 MVA calcium silicate electric furnace
Adding FeO in production to damage the furnace bottom SiC: FeO+SiC=FeSi+CO ↑
During production, small layering is used to damage the furnace bottom at regular intervals, namely:
Extract the lime from the batch and add it separately to the "crucible" area to achieve the goal of using CaO to destroy SiC: CaO+SiC=CaSi+CO1
In appropriate circumstances, it is possible to switch to FeSi production for a few days, and then convert to CaSi after the furnace lump decreases. The effect is good, but there are many intermediate products.
5.3 Maintenance and use of tapping holes
Due to the blowing of oxygen and the erosion of a large amount of calcium silicate slag during the tapping process, the amount of damage to the tapping port is large, causing difficulty in blocking eyes and even endangering the furnace life cycle, requiring regular maintenance. In order to extend its service life, daily maintenance is very important.
(1) Add more carbon materials to the blocked mud balls to reduce the oxidation of the furnace mouth caused by oxygen blowing and suction.
(2) Try to use a steel chisel to pierce through the furnace during discharge to reduce the oxidation of the furnace hole caused by the use of oxygen.
(3) Minimize the occurrence of eye running accidents as much as possible, as the large amount of amphoteric oxide slag during eye running can greatly damage the furnace eye.
(4) Special materials such as carbon magnesium bricks, alloy steel jade bricks, graphite, etc. can be used to make iron mouths, but the cost is relatively high and can be determined based on one's own technical and economic indicators.
5.4 Selection of Polar Circle Diameter
When selecting the diameter of the pole center circle, theoretical calculations cannot be relied solely on. Due to the different short network design and electrical losses of each furnace, the effective power input into the furnace cannot be the same. Therefore, when selecting the diameter of the pole center circle, it should also be determined based on the gradient of the effective phase voltage. For example, the comparison of the pole center circle before and after the adjustment of the effective phase voltage for furnace 1 * of Sichuan CaoDian Silicon Calcium Plant in April 2002 is shown in Table 3.
Technological innovation
Honesty is the foundation
Contact Number: +86-15698999555 |
Address: NO.6 ,SHENGHUA STREET,TAIHE DISTRICT, JINZHOU CITY, LIAONING PROVINCE, CHINA. |