1、 Introduction
The increasing demand for new inorganic materials in modern technology has greatly promoted the progress of inorganic synthesis technology. Chemical vapor deposition (CVD) is a new technology developed in recent decades for the preparation of inorganic materials. Progress has been made in the study of its thermodynamics and growth kinetics . This method is relatively simple in equipment, easy to operate, and has good process reproducibility. It has been widely used in material purification, new crystal development, thin film material preparation, and semiconductor device development .
Transition metal nitrides mostly exhibit metallic luster in solid state; The d-orbitals of metals can overlap with each other and have conductivity similar to metals, hence they are also known as metal type nitrides. They mostly form interstitial compounds with high melting points and hardness, and have special optical and electrical properties. They are widely used in various fields such as high-temperature materials, anti-corrosion and wear-resistant coatings, catalysis, etc.. Titanium nitride (TiN) is a good conductor of heat and electricity, and is found in 4 At 5K, it exhibits superconductivity; It has high chemical and thermodynamic stability and special mechanical properties , and is commonly used as a surface protective layer for hard alloy cutting tools to increase their wear resistance and service life. The color of T iN is very similar to gold, and it can also be deposited on jewelry and lamps as decorative coatings, which is both beautiful and wear-resistant , and antioxidant . In the field of electronic devices, TiN can also be used as a diffusion film between silicon and metal . Recently, it has also been found that T iN has high biocompatibility and broad application prospects in the medical field , such as being used as an inorganic coating for artificial joints . There are also relevant literature reports on the spectral properties, formation mechanism, and other special properties of TiN.
The traditional synthesis method of metal nitrides is to react with metal elements and N2 or NH3 at a high temperature of 1200 ℃. CVD, as a new technology for material preparation, has been applied in the preparation process of this type of compound. This article takes the CVD preparation of metal nitride TiN as an example to review the latest progress of CVD in this field.
2、 CVD Preparation of TiN
1 CVD Preparation of TiN under Traditional Conditions
The commonly used precursors of CVD include hydrides, chlorides, oxides, sulfides, metal organic compounds , etc. The traditional CVD method uses TiCl4 as the source material and deposits TiN in a mixed gas system of N2 or N2 H2. The research on this system is relatively mature:
T iCl4 (g)+2H 2 (g)+1/2N 2 (g) →
T iN (s)+4HCl (g) $fH m (brush)=2 88kJ. m o l -1
This reaction is endothermic. When the system temperature increases, it is thermodynamically beneficial for the formation of TiN. However, in different CVD devices (hot wall or cold wall reactors), the trend of reaction rate changing with temperature varies. Kato and Tamari studied the effects of various growth conditions such as temperature, gas partial pressure, carrier gas flow rate, and substrate on the morphology and growth rate of TiN crystals, and prepared needle shaped TiN crystals within the range of 1200 to 1300 ℃. In their work, it was also found that T iN preferentially grows in the<111>direction, which is consistent with the later research conclusions of Yokoyam , Bu it ing, etc., that the preferred crystal orientation and deposition temperature of TiN are related to the partial pressure of T iCl4. When there is a high partial pressure of T iCl4 at 500 ℃, the main crystal orientation of TiN is<200>; When the partial pressure of T iCl4 is low above 700 ℃, the main crystalline orientation of TiN is<111>. When the temperature is above 1000 ℃, there are reduction products T iCl3 and T iCl2 of T iCl4 in the gas phase. Based on this, Kato et al. speculated on the mechanism of the above reaction as follows:
TiCl4 (g)+1/2H 2 (g) → T iCl3 (g)+HCl (g)
TiCl4 (g)+H 2 (g) → TiCl2 (g)+2HCl (g)
H 2 (g) → 2H (ad)
TiCl2 (g) → TiCl2 (ad)
TiCl2 (ad)+H (ad) → TiCl (ad)
2 CVD Preparation of TiN under Different Source Materials
In plasma enhanced chemical vapor deposition (PECVD) or plasma assisted chemical vapor deposition (PACVD) ,systems, the reaction temperature between TiCl4 and N2 can be reduced to 200-400 ℃, and the carrier gas can be induced to decompose into sediment in the plasma atmosphere, expanding the range of deposited materials. However, in large-scale PECVD reactions, the plasma generated by the initial discharge has poor conductivity, and there are often problems such as uneven product deposition. High voltage stable pulse discharge is needed to solve the problem of poor plasma conductivity. There is a covalent triple bond in the N2 molecule with a high bond energy (941.69 kJ • mol -1), and the N-N bond can only be broken at high temperatures. If other nitrogen-containing substances with stronger reactivity are used as nitrogen sources, the reaction temperature can be reduced to a certain extent. Using other titanium containing substances without chlorine as titanium sources can eliminate the negative effects of chlorine on the reaction. Therefore, while various physical assisted CVDs continue to develop, chemists have been committed to selecting more suitable source materials to optimize reaction conditions.
(1) CVD preparation of TiN under different nitrogen sources
Substances that have been used as nitrogen sources include N 2, NH 3, N 2H 4, CH 3NHN H 2, (CH 3) 2NNH 2, NH 2CH 2CH 2NH2, (CH 3) 3CNH2, azides, acetyl nitrogen, etc. At present, the most commonly used nitrogen source for CVD preparation of nitrides is NH3. Kurtz and Go rdon found that in the system using TiCl4 as the titanium source, the introduction of preheated NH3 can reduce the reaction temperature and increase the reaction rate. Some substrates that are unstable at high temperatures, such as glass and non-metallic silicon wafers, can be applied to this reaction system:
6T iCl4 (g)+8NH 3 (g) →
6T iN (s)+24HCl (g)+N 2 (g)
This reaction can occur at 320 ℃, but usually still occurs above 600 ℃ to improve material properties. Kim and his colleagues first pretreated the substrate with NH3 plasma atmosphere in the PECVD system; Price et al. used more reactive CH 3NHN H2 and (CH 3) 3CNH2 instead of NH3, both of which significantly reduced the reaction temperature. However, due to the fact that TiCl4 is still used as the titanium source in the above work, the influence of chlorine still exists. The doping of chlorine not only causes
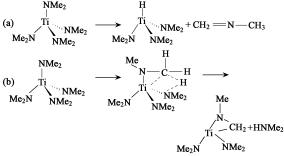
Fig. Po ssible m echanism about tran sam ination reaction: (a) B-elim ination m echanism;
(b) in sertion m echanism
TDMA T During thermal decomposition, there is a characteristic peak of T i-C-N ring in the infrared spectrum at 1276 cm-1, indicating the presence of T i-C-N ring. The presence of T i-C bonds increases the carbon content in T iN. The activation energy of the insertion reaction is greater than that of the elimination reaction. When there is no NH3 in the system, the trend of insertion reaction increases with the increase of temperature, which can explain why the carbon content in TiN increases. Introducing NH3 into the system results in an amino exchange reaction, resulting in a decrease in carbon content. This reaction can be represented by the following general formula:
M (NM e2) 4+2RNH 2 → M (NR) 2+4HNM e2
Pryby la and colleagues demonstrated the reliability of this reaction mechanism using isotope tracing methods:
TDMA T+ND 3+15NH 3 → T i 15N+DNM e2
When Ti [N (CH 3) 2] 4- x (NH2) x [54,62] with a structure similar to TDMA T is used as the titanium source, the carbon content in Ti N decreases. Using T i (N Et2) 4 (TD EA T) [31,63] as a titanium source can increase the stability of T iN and reduce its resistivity, making it more suitable for application in the electronics industry. Applying TD EA T to the PECVD system [64], the growth rate of T iN in the H2 plasma atmosphere is about four times that in the N2 H2 mixed gas plasma atmosphere. T i (NM eEt) 4 (TEM A T) and (acac) 2 T iN Et2 can be decomposed at 250-350 ℃ and 380 ℃, respectively, to obtain T iN, which contains a large amount of carbon deposition. After introducing NH3 into the system, the content of impurities such as carbon and oxygen is greatly reduced. This reaction is similar to the TDMA T and NH3 systems and is also an amino exchange process.
The main intermediate product in the CVD process of preparing T iN from T i (NR 2) 4 and NH 3 systems is the imino (NH=) compound . If T i (NH2) 3NHE t is used as the model for T i (N Et2) 4, the decomposition reaction can go through the following steps:
T i (NH 2) 3NHE t → T i (NH 2) 3NHE t (transition
3、 Outlook
In general, the number of source substances used in CVD reactions is consistent with the number of types of constituent elements in the material. When the material composition is complex, the problem of equipment and operation complexity arises. Single source precursor (SSM p recursor r) contains all the elements that need to be deposited in a single molecule, and its application in CVD systems can simplify the device and facilitate the control of airflow and temperature. Choosing appropriate source materials (single or multiple sources), designing reasonable deposition reactions, reducing reaction temperature without changing the excellent performance of materials, and optimizing reaction conditions have always been the focus of research.
At present, gases such as NH3, H2S, H2Se, and AsH3 are commonly used in CVD processes, which are either toxic, corrosive, or sensitive to air and humidity. Therefore, the search for safer and more environmentally friendly production processes and research on tail gas treatment are of particular significance in today's increasingly prominent environmental issues.
The use of efficient and stable catalysts to promote the CVD process, template method to prepare materials with special morphology and structure, combined with physical methods, to prepare new materials under low temperature and high vacuum conditions has also become the direction of future chemical vapor deposition technology development.
Technological innovation
Honesty is the foundation
Contact Number: +86-15698999555 |
Address: NO.6 ,SHENGHUA STREET,TAIHE DISTRICT, JINZHOU CITY, LIAONING PROVINCE, CHINA. |