1 Introduction
Vanadium is an important alloying element in steel production. Currently, 80% to 90% of vanadium is used in the steel industry mainly because it reacts with carbon and nitrogen to produce melting resistant carbon and nitrogen compounds. Adding vanadium to steel can play a role in grain refinement and precipitation strengthening, improving the comprehensive mechanical properties of steel such as wear resistance, toughness, strength, ductility, and fatigue resistance. Compared with ferrovanadium, vanadium nitrogen alloy can save 20% to 40% vanadium in high-strength low alloy applications, greatly reducing alloying costs. Therefore, vanadium carbide and vanadium nitride alloys are widely used in structural steel, tool steel, pipeline steel, steel bars, ordinary engineering steel, and cast iron. Its good economic and practical value has long attracted the attention of researchers.
Vanadium nitride alloy is actually a solid solution system of vanadium nitride and vanadium carbide, with the chemical formula VC ₁ - N. Both vanadium carbide and vanadium nitride have a face centered cubic structure, which can infinitely solve with each other. The lattice constants are avc=0.4165nm and ayv=0.4137nm, respectively. There are many preparation methods studied at home and abroad, ranging from 16 to 9. Wang Gonghou from Beijing University of Science and Technology used V ₂ Os and activated carbon to first reduce to form VC in a high-temperature vacuum molybdenum wire furnace at 1673K and 1.333Pa vacuum, and then introduced nitrogen gas at a nitriding temperature of 1400 ℃. Samples (86% V-2.7% C-9.069% -9.577% N-2% O) were obtained. United Carbide Corporation of the United States uses high valent vanadium oxide as raw material to produce vanadium nitride by introducing a mixed gas (N ₂+NH Å or N ₂+H ₂), which is then mixed with carbon materials and subjected to high-temperature treatment in an inert or nitrogen atmosphere in a vacuum furnace to obtain 7% vanadium nitride. These methods all have drawbacks such as complex processes, difficult process control, and high production costs. With the increasing research on the production of microalloyed steel in recent years, the author has prepared vanadium nitride alloy blocks with high nitrogen content using more economical and simple processes, and focused on studying the effects of different reaction temperatures on the phase composition and nitrogen content of the products, providing a theoretical basis for formulating the optimal preparation process.
2 Experiments
2.1 Raw Materials and Sample Preparation
This experiment uses industrial grade V ₂ O Å and carbon black as the main raw materials, and adds a small amount of iron powder of about 1% as a sintering additive. The prepared raw materials are placed in a ball milling tank and mixed for 24 hours through rolling ball milling. After being extracted, they are dried at 100 ℃ for 4 hours, and pressed into a size of φ 1.500cm × A 1.070cm cylindrical billet is sintered in a vacuum carbon tube furnace, and N ₂ is introduced to 101kPa (micro positive pressure) under certain reaction temperature conditions. The carbon nitride reaction is carried out simultaneously to prepare a dense vanadium nitride alloy column.
2.2 Sample Phase Composition and Component Analysis
The phase analysis of the sintered products was conducted on a Dandong Fangyuan DX-2000 X-ray diffractometer, with a tube pressure of 40kV and a current of 25mA, CuKa, λ= zero point one five
3 results and discussion
3.1 Thermodynamic analysis of carbon thermal nitridation reaction process
The oxides of vanadium, from high to low valence, are V ₂ O Å, V ₂ O ₄, V ₂ O Å, and VO. According to its oxygen potential, the reduction of V ₂ O Å by carbon heat is stepwise, with V ₂ O Å being the most easily reduced and VO the most difficult. In the actual reaction process, due to the low melting point (940K) and toxicity of V ₂ O Å, in order to reduce the loss of vanadium, the initial reduction starting temperature should be below the melting point of V ₂ O Å, that is, before V ₂ Os transform into a liquid phase, they should be reduced to V ₂ O Å (1633K) with a higher melting point. The reaction mechanism of V ₂ O Å being gradually reduced by C and undergoing simultaneous carbon nitride reaction at high temperatures is very complex, and the reaction process will produce many intermediate phases. The main reaction equations discussed here are:
V ₂ Os (s)+C (s)=2VO ₂ (s)+CO (g) 1
2VO ₂ (s)+C (s)=V ₂ O Å (s)+CO (g) pieces
V ₂ O Å (s)+5C (s)=2VC (s)+3CO (g) t (1)
V ₂ O Å (s)+3C (s)+N ₂ (g)=2VN (s)+3CO (g) T (2)
(1-x) VC (s)+xVN (s)=V (C ₁ - N ₂) (s) VN (s)+C (s)=VC (s)+1/2N ₂ (g) 1 (3)
Based on thermodynamic data [], the Gibbs Helmhotz formula is introduced: △ G Å r=△ H Å 298-T △ S Å 298, and reaction equations (1), (2), and
(3) Δ G Å r, in kJ/mol, i.e
△ G Å r=655500 475.68T (4)
△ G Å r=430420-329.98T (5)
△ G Å r=112549 72.84T (6)
When calculating (4), (5), and (6) in standard state, it can be seen that when Δ G ⁹ r=0
Ti=1378K, T2=1304K, T ₄=1545
3.2Effect of reaction temperature on phase composition and composition of product
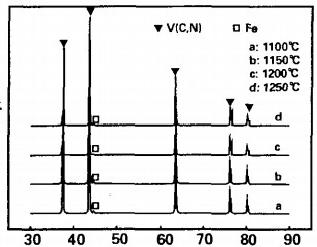
Fig 1 XRD patterns of products at different reaction temperatures
As shown in the figure, when the reaction temperature is within the range of 1100~1250 ℃, the products are composed of V (C, N) solid solution and trace amounts of α- As the reaction temperature increases, the X-ray diffraction peak of the alloy shifts towards a higher angle direction, that is, from the diffraction peak close to the VC characteristic (left) to the VN characteristic (right), indicating a decrease in nitrogen content in the product solid solution. According to the Bragg equation, the lattice constant of the alloy product is increasing. Figure 2 shows the relationship between the theoretical calculation of the xx content in the alloy and the lattice constant of the alloy solid solution with the reaction temperature. It can be seen from the graph that as the reaction temperature increases, the overall trend of nitrogen content change decreases, with a rapid decrease in the range of 1100 to 1150 ℃. When the reaction rises to around 1100 ℃, as the carbon nitride reaction proceeds, the nitrogen content in the vanadium nitride alloy remains high. Thermodynamic analysis shows that the V-C bond bonding temperature is higher than the V-N bond bonding temperature. Therefore, VC ₁ N with high nitrogen content can be prepared at a relatively low temperature of 1100 ℃, alloy, xx=0.67. As the reaction temperature continues to rise, the generated vanadium nitride in the product will gradually be reduced by carbon to form vanadium carbide. The nitrogen content in the product decreases, which means that C atoms replace the N atoms in the lattice of V (C, N) solid solution, resulting in a decrease in nitrogen content in V (C ₁ - N:) solid solution [12]. At the same time, due to the larger atomic radius of C (rc=0.091nm) compared to N (ry=0.075nm), it also leads to an increase in the lattice constant of the alloy, This is also the reason why the diffraction peak in the XRD pattern shifts towards the high angle peak direction. Therefore, in order to produce VN alloys with high nitrogen content, the reaction temperature should be controlled between 1100 and 1150 ℃.
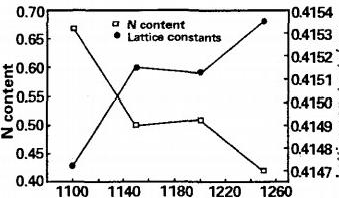
Fig 2 Effect of reaction temperature on N content and lattice constants
3.3 Effect of reaction temperature on product density
Density tests were conducted on alloy cylinder specimens sintered at different temperatures, and the results are shown in Figure 3. It can be seen that the alloy densifies rapidly within the range of 1100~1200 ℃, and the density continues to increase as the temperature continues to rise. In a heterogeneous solid-phase sintering system, solid-phase reactions occur at the phase interface to generate intermediate products, which are then separated from the interface through interface analysis and diffusion. Improving the interface adsorption can improve the reaction activity. However, iron powder, due to its large specific surface area, can adsorb more reactants and cause lattice distortion inside the reactants, reducing the reactant activation energy and increasing the activity, thereby promoting the progress of carbon nitride reaction. When the sintering temperature is low, the atomic diffusion on the surface layer of the reactant particles leads to particle rearrangement and the growth of the sintering neck, resulting in rapid densification (13). As the sintering temperature increases, the atomic diffusion coefficient within the particles continuously increases, resulting in better sintering properties of the particles and a denser sintered body.
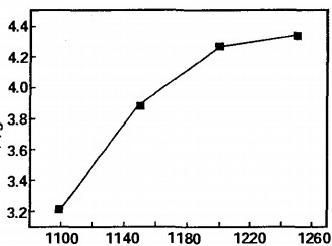
Fig 3 Effect of reaction temperature on density of products
3.4 Chemical composition analysis of samples
The vanadium nitride alloy sample prepared by sintering at 1100 ℃ and holding for 1 hour, with 1% iron powder added as the sintering additive, was analyzed for element content, as shown in Table 1. In the preparation of vanadium nitrogen alloys, carbon content and oxygen content are important control factors, otherwise it will have a significant impact on product use. In the later stage of the experiment, by continuously optimizing process parameters, the nitrogen content has reached over 15% and the oxygen content has been further reduced. It can be seen that by accurately proportioning the raw materials and selecting appropriate process parameters, vanadium nitrogen alloy bulk samples with excellent composition that meet the production requirements can be prepared.
Table 1 Chemical composition of the product
Sample | Temperature(℃) | Flux | element | |||
V | C | 0 | N | |||
VN-1 | 1100 | 1%Fe | 76.58 | 5.41 | 1.46 | 16.2 |
4 Conclusion
The thermodynamic calculation provides a theoretical basis for determining the heat treatment temperature of the VN alloy prepared by the thermal reduction nitridation reaction of V ₂ O Å with carbon black. Sintering at 1100 ℃ and holding for 1 hour, adding 1% iron powder as a sintering additive to the raw material can prepare a dense vanadium nitride alloy sample with high nitrogen content. The nitrogen content of the product decreases with the increase of sintering temperature, while the density continuously increases.
Technological innovation
Honesty is the foundation
Contact Number: +86-15698999555 |
Address: NO.6 ,SHENGHUA STREET,TAIHE DISTRICT, JINZHOU CITY, LIAONING PROVINCE, CHINA. |