Metal carbides, as a new type of functional material with high melting point and hardness, good thermal and mechanical stability, and excellent corrosion resistance, have been widely used in various fields such as high temperature resistance, wear resistance, and chemical corrosion resistance. In addition to possessing the characteristics of metal carbides mentioned above, molybdenum carbides have also been found to have electronic structure and catalytic properties similar to precious metals. They can be widely used as catalysts for reactions involving hydrogen, such as alkane isomerization, unsaturated hydrocarbon hydrogenation, hydrodesulfurization, and denitrification. Below is a brief overview of the properties, applications, and preparation methods of molybdenum carbide.
1.Preparation of Molybdenum Carbide by Mechanical Alloying
1.1 Preparation of Micro Nano MoC Powder
Experimental method:
Vacuum the high-energy ball mill, continuously introduce protective gas and reaction gas CO, and use zirconia ceramic balls as the milling medium with a ball to material ratio of 8:1. The milling time is 30 hours, and the average diameter of the obtained MoC powder is 100nm.
Test results:
The final product obtained from ball milling is MoC with a hexagonal structure. Before ball milling, the XRD curve is a set of standard diffraction spectra of Mo. In the early stage of ball milling, only the broadening of Mo's diffraction peak is caused. After 10 hours of ball milling, a solid gas chemical reaction between Mo and coal gas caused by mechanical energy begins to occur, with a small amount of MoC formed. As the milling time increases, the content of MoC also increases continuously. After 30 hours of ball milling, almost all diffraction peaks of the original powder Mo disappeared, replaced by a set of diffraction spectra of MoC with a hexagonal structure. The original molybdenum powder particles have significant differences in size. As the ball milling time increases, the powder particles gradually become smaller and more uniform. After 4 hours of ball milling, the powder particles are smaller and have a uniform size distribution compared to the original powder. After 16 hours of ball milling, due to further refinement of the particles, the powder exhibited agglomeration phenomenon. After 30 hours of ball milling, the average particle size of the powder approached the nanometer level, while some agglomerated particles still appeared. The particle size of the powder measured below. After 4 hours of ball milling, the particle size distribution of the powder is relatively uniform, and the particle size is also significantly reduced, with an average particle size of around 400nm. After 30 hours of ball milling, the powder became more uniform and fine, with an average particle size of around 100nm.
Discussion:
1) The experimental results show that through high-energy ball milling of metal powder in gas atmosphere, metal carbonization reaction can be achieved at room temperature, and submicron level metal carbide powder can be formed. This technology provides a new approach for the synthesis of submicron metal carbide powders. The mechanochemical reaction process is caused by the action of mechanical force to change the properties and structure of the powder. Mechanical energy causes the powder to undergo crystal transformation and induces chemical reactions. It is generally believed that during high-energy ball milling, powder particles are in a high-energy state. At the moment of collision between the ball and powder particles, a highly active zone is formed, and a temperature rise is generated, which can induce chemical reactions in this area. As the ball milling process continues, fresh surfaces are continuously produced, thereby maintaining the progress of the reaction. In the process of mechanical chemical reaction between Mo and coal gas to generate submicron molybdenum carbide, energy is an important factor affecting the overall rate of mechanical chemical reaction. The adsorption and dissociation of C atoms in gas atmosphere require a certain amount of energy, and the transformation of Mo-C chemical adsorption to Mo-C carbides also requires a certain amount of energy activation; In addition, the refinement of the original molybdenum powder particles and the further refinement of the generated molybdenum carbide powder particles also require energy maintenance. Therefore, to ensure the progress of mechanochemical reactions, sufficient energy must be provided by the external environment. In this experiment, energy is transmitted to the powder through the rotation of the stirring shaft, which drives the collision between the medium ball and the powder. Therefore, the amount of energy provided mainly depends on the speed of the stirring shaft, and different speeds provide different mechanical energy.Under the X-ray diffraction of samples with different ball milling times at a lower ball to material ratio of 8:1, after 30 hours of ball milling, the average particle size of the powder is around 100nm.
2) In the high-energy mechanochemical reaction process between Mo and gas atmosphere, it can be seen that although the speed difference is 200r/min, it has a significant impact on the mechanochemical reaction process. At this relatively low speed, the solid-state to gaseous chemical reaction between Mo and gas does not begin until 12 hours after ball milling. The mechanical and chemical reactions after 22 hours of ball milling are comparable to those after 16 hours of high-speed ball milling. After 30 hours of ball milling, the solid gas chemical reaction was basically completed at high speed, while the reaction at low speed was only about 86% complete. Therefore, in the high-energy mechanochemical reaction process of Mo under gas atmosphere, the rotational speed has a significant impact on the reaction rate.
Conclusion:
1) This experiment uses a self-designed high-energy mechanochemical ball mill to obtain molybdenum powder through high-energy ball milling under gas atmosphere at room temperature. The speed of ball milling plays an important role in the reaction speed.
1.2 Ultra fine Mo2C and MoC
Test method:
Zhang Qiaoxin et al. successfully prepared ultrafine Mo2C and MoC compounds by mixing molybdenum powder and carbon black powder in atomic ratios of 2:1 and 1:1 using mechanical alloying.
Experimental discussion:
The MA process and products of the alloy are closely related to the ball to material ratio. When the ball to material ratio is 50:1, both alloys exhibit three stages: mixing, amorphization, and crystallization; 20: At 1 hour, there was no such phenomenon, but the products at both ball to material ratios were MoC phase; At 70:1, the product of 2Mo-C alloy is Mo2C phase.
Application examples:
One of the applications of MoC metal ceramic powder is that it can be used as a structural coating material. Therefore, plasma spraying method is used to form a metal ceramic coating on the substrate material. The bonding metal is commonly used Ni60 metal alloy spraying powder, in which different mass fractions of micro nano MoC powder are added. The sprayed powder is coated on the substrate material using plasma spraying equipment. The substrate material is 40Cr steel, and the wear test of the coating shows that the wear resistance of the coating has been greatly improved. In addition, it is also widely used in fields such as machinery, aerospace, petrochemical, and electronic appliances.
Innovation highlights:
Firstly, the use of mechanical alloying to produce molybdenum carbide can be carried out at room temperature, which greatly saves energy and is a worthwhile research direction. Secondly, the molybdenum carbide powder obtained by this method is relatively fine, has a high specific surface area, and has great application prospects.
2.Preparation of molybdenum carbide by gas-phase method
2.1 Preparation: The gas-phase method generally uses high specific surface area activated carbon (with a specific surface area greater than 200 ㎡/g) and transition metal volatile compounds, which are stoichiometric and fed into a flowing reactor. The reaction is carried out at a high temperature of 900-1400 ℃ for a certain period of time, cooled in an inert gas, and the carbides are recovered. Heat a mixture of high specific surface carbon and MoO3 in argon gas to 800 ℃, sublimate and adsorb MoO3 onto the carbon, and heat it up to 1300 ℃ for a certain reaction time to obtain Mo2C. There are reports that a hexagonal Mo2C system can be obtained by reacting MoO3 with 1150 ㎡/g carbon in a C/Mo molar ratio of 6:1, with a specific surface area of 213 ㎡/g. The chemical vapor deposition method obtains carbides by vaporizing molybdenum metal oxides or metals at high temperatures and then reacting with the carbonized gas.Leclercq et al. evaporated volatile metal compounds into containers containing low-pressure carbon hydrides and directly carbonized them at high temperatures to produce carbides. Different carbides and carbon oxides are produced by changing the gas composition in the reactor and the types of metal compound precursors. Nagai et al. used this method to prepare Mo2C/Al2O3 catalysts with activity three times higher than the impregnation method under 973K vacuum conditions. But the disadvantage of this method is that the conditions are difficult to control and the synthesis amount is small.
2.2 Application: The molybdenum carbide produced by this method is widely used in the field of material modification, mainly due to its high melting point and hardness, good thermal and mechanical stability, and excellent corrosion resistance. It can be used as a coating material or as an additive material. In addition, molybdenum carbide can be mixed with tungsten carbide, added with appropriate lanthanides, and sintered into a hard alloy production method, which can obtain molybdenum carbide based hard alloys with good bonding phase distribution, density, and refinement. Molybdenum carbide can also be added to metal ceramics to improve their performance. In addition, molybdenum carbide is commonly used in particle reinforced alloys.
2.3 Analysis: Compared with previous high-temperature heating methods, the vapor deposition method has improved in terms of increasing specific surface area. However, the drawback of this method is that the preparation process requires a high temperature and cannot be used for coating complex shaped components and fine materials, requiring expensive equipment, etc.
3.Preparation of molybdenum carbide by 3-ion melting method
3.1 Preparation: The ion melting method involves electrolyzing molybdate and carbonate in an electrolytic container, and forming a molybdenum carbide coating through co deposition of molybdenum and carbon during electrolytic reduction. Under optimal conditions, a smooth coating with a thickness of 100um can be formed on the metal. Thermodynamic analysis shows that sodium molybdate, lithium molybdate, sodium carbonate, and lithium carbonate have the closest decomposition potential energy. Molybdenum and carbon can co deposit and form carbides in electrolytic reduction. Electrolysis is carried out in a MYIF-7 graphite container. To prevent oxidation of the container, it is placed in a corundum test tube with argon gas flow and a solution is prepared using a pre dried reagent. The electrolyte is subjected to electrolysis treatment in the negative electrode current range of 1.0~1.5 · 102A/㎡ in advance, which can stably and continuously obtain the precipitate. The negative electrode of conductive metals uses graphite, nickel sheets, copper sheets, and steel sheets. Non conductive materials - dispersed diamond, silicon carbide, and boron carbide grinding powders are packed in nickel containers. The positive electrode uses a graphite container.
3.2 Application: The deposition of molybdenum carbide coatings on metal materials, diamond, silicon carbide, and boron carbide particles using ion melt electrolysis method. Electroplating can improve the wear resistance, grinding and destructive properties of metal materials, enhance their wettability, and enhance the efficiency of diamond, silicon carbide, and boron carbide. Saves superhard materials.
3.3 Analysis: The ion melting method compensates for the high temperature required in the preparation process of the vapor deposition method, which cannot be used for coating complex shaped parts and fine materials, and requires expensive equipment. The coating speed of this method mainly depends on the electrolysis temperature and negative electrode current. The production temperature of dense Mo2C precipitate is 8OO-950 ℃ (the higher the temperature, the tighter the particles in the precipitate). But at 800 ℃, the thickness of the carbide layer is less than 10um, and the strength of the precipitate bonding with the bottom plate is very low; At a temperature of 750-800 ℃, powdery carbide residues will peel off. The electrolysis conditions have an impact on the precipitate and structure of molybdenum carbide: a coating with good adhesion, uniform density, and no porosity is obtained at a negative electrode current density of 1.0 × 102-1.0 × Made under the condition of 103A/㎡. In the initial stage of precipitation, the fewer crystals in the precipitate, the higher the current density. When the current density is high, it can be observed that a large number of particles merge, leading to an increase in the roughness of the coating. At a current density of 1.0 × At a rate of 102A/㎡, the roughness of the coating decreased, obviously due to a weakened bonding force. When the current density is greater than 10 × At 102A/㎡, the corrosion rate of the bottom plate is higher than that of the precipitated crystals, and a coating with good adhesion cannot be obtained. In addition, the concentration of reactants also affects the preparation of molybdenum carbide. So, strict control of reaction conditions is crucial.
4.Preparation of molybdenum carbide by impregnation method
4.1 Preparation: Carbon supported molybdenum precursor prepared by impregnation method: Vulcan XC-72 carbon black (VC) was impregnated with a measured amount of acetylacetone molybdenum MoO2 (acac) 2 (Sigma Aldrich) aqueous solution After stirring and soaking at 80 ℃ for 5 hours, carbon supported molybdenum precursor MoO2 (acac) 2/VC was prepared by drying at 110 ℃ for 12 hours Fill 0.2 g of MoO2 (acac) 2/VC into a vertical quartz tube furnace, and raise the temperature from room temperature to 800 ℃ at a rate of 5 ℃/min under an argon atmosphere, and maintain the temperature at 800 ℃ for 2 hours Then slowly cool to room temperature to obtain the catalyst, denoted as Mo2C/VC.
4.2 Application: Mo2C/VC has catalytic performance similar to Pt/C in fuel cells, which can be used not only for hydrogen electrocatalytic oxidation reactions, but also for cathodic oxygen reduction reactions. In addition, molybdenum carbide has been applied in processes such as catalytic hydrogenation, catalytic dehydrogenation, catalytic hydrodesulfurization, catalytic hydrogenation denitrification, isomerization, ammonia decomposition, and aromatization.
4.3 Analysis: The bulk phase of Mo2C/VC samples is β- Mo2C Due to the high reactivity of Mo2C with oxygen and the inability to completely remove oxygen during the Mo2C preparation process, the surface structure and bulk phase of the sample may be different, resulting in the adsorption of oxygen on the surface and the formation of oxides The electrocatalytic performance of Mo2C/VC may be caused by the oxidation-reduction of surface passivation species (MoOxCy and MoOz), as well as the migration of lattice oxygen in and out of Mo2C.
In addition, carbides and even nanoscale particles can be obtained through plasma sputtering method. The research on molybdenum based nanomaterials has created a vast space for the new application of molybdenum. Compared with traditional "bulk" molybdenum materials, nanomaterials have unique properties and are widely used. For example, nano molybdenum fertilizer can penetrate various parts of plant roots, making it highly efficient and long-lasting.
5.Preparation of Molybdenum Carbide by 5-Program Temperature Reduction Method
The programmed temperature reduction method uses transition metal oxides mixed with hydrocarbons and hydrogen as raw materials, and undergoes a process similar to the programmed temperature reduction process. It can be carried out at a set temperature, allowing the transition metal oxides to undergo a reaction process of reduction and carbonization (carburization). Commonly used hydrocarbons include methane, ethane, and ethylene, and CO and CO2 can also be used as carbon sources. The reaction temperature is generally within the range of 400-1000 ℃, and the specific surface area of the prepared carbides ranges from a few to 200 ㎡/g.
The early synthesis of molybdenum carbide and molybdenum nitride mainly used high-temperature heating reaction method, but the specific surface area of synthesized molybdenum carbide is generally very small.
The temperature programmed reaction method developed in the 1980s opened up a new pathway for the synthesis of high specific surface area carbides and nitrides. In this method, metal oxides are generally used as precursors, and the reducing gas is usually a mixture of 20vo1% CH2-80vo1% H2 or pure NH3. Strictly controlling the heating rate and gas flow rate during the reaction process achieves the optimal balance between the sintering and synthesis rates of the catalyst, resulting in a larger specific surface area of the obtained sample. Volpe J and Oyama et al. used this method to prepare samples with a specific surface area of 50-90 ㎡/g, respectively β- MoC.
5.1 Experiment
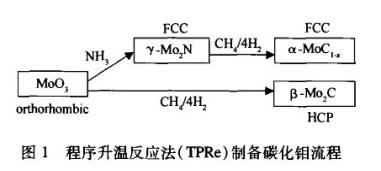
Experiment Example 1: Molybdenum carbide was prepared using the temperature programmed reaction method (TPRe), which involves loading a certain amount of MoO3 into a quartz reaction tube and placing it in a tube furnace. β- The preparation of Mo2C involves direct carbonization using a mixture of n (CH4)/n (H2)=1/4 as the carbonization medium, with a space velocity of around 15000h-1. Adopting a two-stage temperature control program, the heating rate from room temperature to 673 K is 5 K/min, and after 673 K, the heating rate is 1 K/min; And only α- The preparation of MoC1-x is first nitrided with NH3 and then carbonized. Finally, the sample was rapidly cooled to room temperature in Ar, and then passivated using an O2/N2 mixture containing trace amounts of oxygen. The specific process is shown in Figure 1.
Experiment Example 2: Pure Mo2C and supported Mo2C samples were prepared using the programmed temperature reaction method proposed by Bouhart et al. MoO3 is obtained by decomposing ammonium molybdate (A.R.) at 500 ℃ for 4 hours. Alumina supported MoO3 is prepared by impregnating an industrial alumina carrier (20-40 mesh, Fushun Petrochemical Research Institute) with ammonium aluminate (A.R.) - ammonia aqueous solution prepared according to the measurement. It is stirred for 30 minutes and left at room temperature overnight. Then, it is heated to dryness in a 50 ℃ water bath for 4.5 hours, transferred to an oven (110 ℃) for about 13 hours, and then roasted in a muffle furnace at 500 ℃ for 4 hours to obtain the precursor MoO3 of the catalyst. Weigh 2.1 g (approximately 3 mL) of precursor into the reaction tube of the multifunctional catalyst evaluation device (KY-1020D type, Jiangyan Keyuan Electronic Instrument Co., Ltd.), with a hydrogen flow rate of 270.8 mmol/h at atmospheric pressure. According to different carbonization end temperatures and different carbonization feed rates, the Mo2C catalyst for research was prepared by increasing the temperature to the carbonization end temperature at a rate of 1K/min and keeping it constant for 2 hours before rapidly cooling to room temperature. The catalyst was passivated with N2 gas flow containing 1% O2 for 6 hours.
Experiment Example 3: Molybdenum oxide used in the experiment is obtained by roasting ammonium molybdate at 550oC in a muffle furnace for 4 hours; Molybdenum carbide is prepared by pressing and sieving corresponding oxide precursors, and then placed in a self-made reduction carbonization experimental device at a volume space velocity of 6 × Program heating to the given temperature in a CH4/H2 atmosphere for 100 hours, maintain a constant temperature for 3 hours, rapidly cool to room temperature, and passivate at room temperature for 3 hours with N2 gas flow containing 1% O2.

5.2 Analysis
In Example 1:
Schedule for preparing molybdenum carbide from MoO3:
| The first stage of program heating | The second stage of program heating | |
| Heating range | 298k-650k | 650k-950k |
| Speed | 5k/min | 1k/min |
| Time | 70 minutes | 300 minutes |
Example 2:
Schedule used for producing MoO3:
| Time | based MoO3 total time | based molybdenum carbide reaction time | |
| Pure MoO3 | 4h | 4h | heated to 600 degrees Celsius for 10h maintained at a constant temperature for 2h, purified for 6h=18h |
| Aluminum oxide supported MoO3 | 30min+12h+4.5h+13h+4h | 34h |
In Example 3:
MoO3 undergoes temperature programmed reduction carbonization reaction in CH4/H2 atmosphere, which can be converted into Mo2C. The reaction process can be expressed as MoO3 Mo02 Mo0xCy Mo2C, and the appropriate reaction temperature should be around 675oC. The surface Mo/C ratio of the molybdenum carbide sample is 0.95, which is less than the theoretical atomic ratio of Mo2C of 2. This is because there is excess carbon on the surface of the sample, resulting in a low surface ratio.
From the two tables, it can be seen that most of the time is spent on preparing MoO3 during the preparation of Mo2C. In the process of preparing MoO3 through alumina supported MoO3, the time for preparing MoO3 even accounts for 74%. It can be seen that the preparation process of Mo2C is too complicated. Analyzing the products of the experiments in Example 1, Example 2, and Example 3, it is found that the yield of Mo2C is relatively low, and there are many impurities, making purification relatively difficult. It is also difficult to control the surface ratio. So if we want to promote the application of molybdenum carbide, we must solve two problems:
1、 Simplify the preparation of molybdenum carbide raw materials;
2、 Shorten the preparation time of molybdenum carbide and improve the reaction rate.
Improvements can be made in the following areas:
1. Simplify the preparation process of MoO3
2. Searching for Catalysts for MoO3 Preparation of Mo2C Reaction
3. Adopting different preparation methods for different purposes
5.3 Application of molybdenum carbide prepared by programmed heating:
1. The carbide crystals formed on the surface of the carrier in Example 2 are a very thin layer of crystal film, so that the characteristic peaks of the carbides cannot be detected by XRD. This also indicates that the preparation method of the carbon source used in the experiment can produce highly dispersed catalysts.
2. Compared with different preparation methods of molybdenum carbide, the surface of the programmed heating method is generally higher. And the surface ratio can be adjusted according to different ingredient ratios.
3. The program temperature reduction method can be used to prepare Mo2C powder with a specific surface area of 50-100m2/g hexagonal crystal system. Research has shown that different preparation processes can yield MoC1-x (0 ≤ X ≤ 0.5) with different chemical compositions and molybdenum carbide with different crystal states.
5.4 Summary:
The laboratory production of various aluminum carbide catalysts usually adopts the programmed temperature carbonization method developed by Bouhart et al., which can select many raw materials. However, currently the main carbonization raw materials used are a mixture of methane and hydrogen, and there are also a few reports of using ethane, acetylene, propane, and butane. When using these substances as carbon sources, a higher carbonization temperature is usually required, so the production of molybdenum carbide is limited to high-temperature carbides. From the perspective of the formation mechanism of carbides, the process of carbon precipitation after hydrocarbon dehydrogenation has a significant impact on the formation of carbon oxides and carbides, that is, hydrocarbons that are relatively easy to precipitate carbon have a promoting effect on the formation of carbides to a certain extent. In fact, from the results of preparing carbides with different carbon sources, longer chain hydrocarbons exhibit higher reaction activity. Therefore, the temperature required for carbonization is relatively low. Considering the large-scale preparation of the catalyst, using liquid organic raw materials as a carbon source would make the operation much more convenient. At the same time, there is still little research on C6 as a carbonization raw material.
6. Application of Molybdenum Carbide
6.1 Material modification
Molybdenum carbide is applied in the field of material modification mainly due to its high melting point and hardness, good thermal and mechanical stability, and excellent corrosion resistance. It can be used as a coating material or as an additive material.
Molybdenum carbide is commonly used as a coating material in fields such as high hardness, wear resistance, and high temperature resistance.
Molybdenum carbide (MoC) and tungsten carbide are mixed, added with appropriate lanthanum powder, sintered into a hard material. After rough crushing, a certain amount of nickel is added, and the usual hard alloy production method is used to obtain molybdenum carbide based hard alloys with good bonding phase distribution, density, and refinement. Molybdenum carbide can also be added to metal ceramics to improve their performance. In addition, molybdenum carbide is commonly used in particle reinforced alloys.
6.2 Catalytic materials
Molybdenum carbide has certain properties of precious metals, and its catalytic activity for hydrocarbon dehydrogenation, hydrogenation, and isomerization reactions is comparable to that of precious metals platinum and iridium, and is known as a "quasi platinum catalyst". Especially due to its lower price compared to precious metals and excellent sulfur poisoning resistance, it has broad application prospects in the field of catalysts. Molybdenum carbide, as an excellent catalytic material, must have a high specific surface area.
7 Conclusion
Metal carbides are a new type of functional materials with high hardness, high melting point, good thermal stability, and corrosion resistance, which have been applied in many fields. In recent years, carbides, especially molybdenum carbide, have attracted widespread attention from researchers as a new type of catalytic material. In terms of its catalytic properties, molybdenum carbide is similar to platinum group precious metals in many aspects, especially in its hydrogenation activity, which is comparable to precious metals such as Pt and Pd, and is expected to become a substitute for precious metals. The literature indicates that molybdenum carbide exhibits unique performance in many catalytic fields, demonstrating the potential application prospects of this type of catalytic new material.
Technological innovation
Honesty is the foundation
Contact Number: +86-15698999555 |
Address: NO.6 ,SHENGHUA STREET,TAIHE DISTRICT, JINZHOU CITY, LIAONING PROVINCE, CHINA. |