Bu Wenbo, a professor in the Department of Materials Science of Fudan University, has been selected as a highly cited scientist of Kerui Vian Global for four consecutive years. Recently, MB NPs (metal-boride nanoparticles) prepared by him and his team have provided a "treasure house" of materials with great clinical application value in the field of biomedical materials.

Figure | Bu Wenbo (source: Bu Wenbo)
The lattice of active metal boride shows extremely rich bonding modes, which can hydrolyze to produce intermediate borohydride nanosheets and release ions, which endows boride materials with versatility.
In addition, the new strategy of "boron capture" proposed this time reveals that active metal borides can destroy bacterial structure and achieve sterilization by combining with the key polysaccharide components of bacterial cell wall (lipopolysaccharide/peptidoglycan).
The results of a series of comparative experiments show that the bactericidal activity of this strategy is equivalent to that of classical antibiotics such as amikacin, gentamicin and ciprofloxacin, and even better than that of antibiotics such as amtreonam, ampicillin and sulbactam.
It is worth mentioning that active metal borides can reduce the drug resistance of bacteria by destroying the bactericidal mechanism of bacterial structure, and are expected to achieve more long-term bactericidal effect.
Therefore, as an efficient antibacterial component, adding this material to textiles can inhibit the growth of bacteria and prevent the generation of odor; It can also be made into antibacterial coating and applied on the surface of metal implants or medical devices to achieve efficient sterilization.
It is also reported that active metal borides not only have antibacterial function, but also can use the "boron capture" function to combine with the free lipopolysaccharide/peptidoglycan released by dead bacteria to effectively inhibit the excessive inflammatory reaction induced by dead bacteria.
Therefore, this material can also be used for a series of bacterial infection-related diseases, such as skin infection, wound infection and gastrointestinal ulcer, to solve the clinical problem that traditional antibacterial agents are only limited to killing live bacteria and can not inhibit dead bacteria to induce excessive inflammation of the host.
The team further found that the "boron capture" property of active metal borides can affect the function of sugar by complexing key sites of sugar, thus playing an important role in the treatment of complications of diabetes and other diseases of glucose metabolism.
They predicted that the controlled release of ions of this kind of material also has the potential to regulate the ion current, and is expected to play a potentially important role in the field of neurological diseases.
Realize efficient treatment of infected wounds
It is reported that bacterial infection is an important reason for chronic wound healing, which can lead to sepsis, multiple organ failure and even death in severe cases.
At present, antibiotics and antibacterial nano drugs are mainly used in clinical treatment of wound infection. Although these drugs can effectively inhibit the growth of bacteria, the dead bacteria will release a large amount of free lipopolysaccharide or peptidoglycan, which will activate the host immune cells and cause excessive inflammatory reaction, resulting in long-term failure to heal the wound, greatly limiting the therapeutic effect of these drugs.
Therefore, how to make drugs have the dual functions of inhibiting the growth of living bacteria and inducing excessive inflammation by dead bacteria is a scientific problem that needs to be solved urgently.
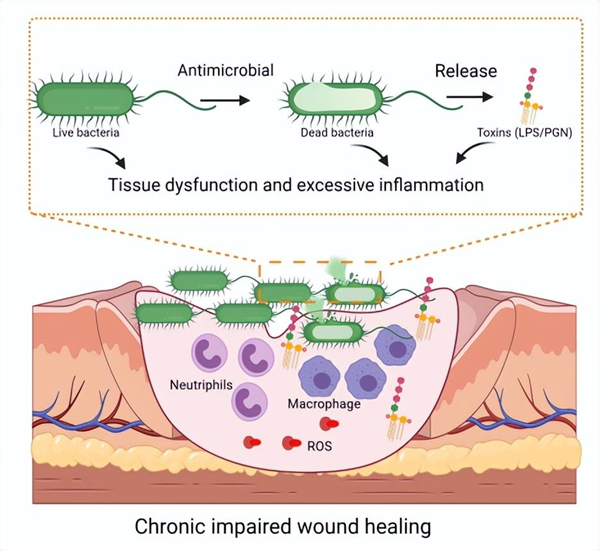
Figure | Schematic diagram of live bacteria and dead bacteria blocking wound healing (source: Bu Wenbo team)
It has been shown that some key components of pathogens play a key role in their structure and function. For example, lipopolysaccharide/peptidoglycan are the key components of gram-negative bacteria and gram-positive bacteria respectively.
On the one hand, lipopolysaccharide/peptidoglycan is the key structural component of bacterial cell wall, which plays a role in maintaining the integrity of bacterial structure and protecting bacteria from the killing of antibacterial agents.
On the other hand, lipopolysaccharide/peptidoglycan is also the main functional component of bacterial toxin, which can be released from the outer membrane of dead bacteria. It also has high immunogenicity, which can induce excessive inflammation in the host and destroy the host tissue.
It is noteworthy that lipopolysaccharide/peptidoglycan contains typical 1,2 - or 1,3-o-dihydroxy groups, which are important structural basis for lipopolysaccharide/peptidoglycan to perform corresponding functions.
Therefore, the team speculated that if the key group of lipopolysaccharide/peptidoglycan was captured by chemical means, it could not only inhibit the viability of living bacteria structurally, but also inhibit the excessive inflammation induced by dead bacteria functionally, thereby achieving the goal of promoting efficient wound healing.
So, how to design new functional materials from the perspective of materials science, and how to capture the key component of bacteria - lipopolysaccharide/peptidoglycan, so that the materials can both inhibit the growth of living bacteria and inhibit the excessive inflammation induced by dead bacteria, is the key to achieve efficient treatment of infected wounds.
Based on this, the team first thought of borate materials, which are rich in boron dihydroxy groups and can react with the adjacent dihydroxy groups of sugar to form dynamic borate ester bonds. This dynamic covalent bond has been widely used to identify substances such as blood support, glucose and adenosine triphosphate.
Unfortunately, the borate ester bond is very easy to dissociate under acidic and inflammatory conditions, and it may be that borate materials are not suitable for such disease models.
For this reason, the team further considered whether it could improve the stability of borate ester bonds in the pathological microenvironment by designing materials?
After investigating the classical reaction of "boric acid-sugar complexation", they found that the stability of borate ester bond was closely related to the configuration of boron element.
Under alkaline conditions, the configuration of boron can be changed from sp2 to sp3. This process can cause the change of the bond angle of the boron dihydroxy group, thus effectively releasing the bond tension of the cyclic borate ester bond, thus improving the stability of the borate ester bond.
Based on the above principles, the team believes that the design of materials that can form stable borate ester bonds will be the key to efficiently capture lipopolysaccharide/peptidoglycan.
Based on the above background, they designed and synthesized this kind of active metal boride, which can hydrolyze to generate boron dihydroxy and hydroxide, and release metal cations.
Among them, the alkaline microenvironment created by hydroxyl radical can change the configuration of boron atom from sp2 to sp3, thereby promoting the esterification reaction between boron dihydroxy group and adjacent dihydroxy group of sugar, thus forming stable borate ester bond.
This also means that the lipopolysaccharide/peptidoglycan on the surface of bacteria can be captured by using this new mechanism of "boron capture": on the one hand, it can sterilize by destroying the structure of living bacteria, on the other hand, it can neutralize the toxin released by dead bacteria, and finally effectively promote the healing of infected wounds.
Through this strategy, they solved the clinical bottleneck problem that traditional antibacterial agents were only limited to killing live bacteria, but could not inhibit the excessive inflammation induced by dead bacteria.

Figure | Schematic diagram of antibacterial and anti-inflammatory effects using "boron capture" mechanism (source: Bu Wenbo team)
However, the question follows. How can active metal borides achieve antibacterial and anti-inflammatory effects after capturing lipopolysaccharide/peptidoglycan on the surface of bacteria?
Research on molecular biological mechanism shows that the combination of active metal borides and lipopolysaccharide/peptidoglycan on the bacterial surface can significantly increase the concentration of local cations on the bacterial surface, such as Mg ions, which can change the membrane potential of the bacterial outer membrane, thereby damaging the permeability of the membrane, finally activating the bacterial RNA degradation signal pathway, and playing the role of efficient sterilization.
On the other hand, the combination of active metal borides and free lipopolysaccharide/peptidoglycan released by dead bacteria can effectively inhibit the phosphorylation of MAPKs induced by free lipopolysaccharide/peptidoglycan, including P38, Erk and JNK signal pathways, thus inhibiting the inflammatory reaction.
A series of in vivo experiments showed that the active metal boride has the ability to significantly promote the healing of infected wounds in mice. It can be said that this study not only solved the key bottleneck problem that dead bacteria are easy to cause excessive inflammation, which was ignored in previous studies, but also creatively revealed the new mechanism of active metal borides in antibacterial and anti-inflammatory aspects, which is expected to provide new ideas for the development of new antibacterial and wound healing agents and the treatment of clinical anti-infectious diseases.
To sum up the above, Bu Wenbo's team summarized this kind of active metal boride material as "boron magnetic" material, put forward the strategy of "boron magnetic" to capture the key components of bacteria, and revealed its antibacterial and anti-inflammatory functional mechanism, which can achieve efficient treatment of infected wounds.
Recently, relevant papers were published on Nature Communications with the title of "Reactive metal boride nanoparticles trap lipopolysaccharide and peptidoglycan for bacterial infected wound healing".
(Source: Nature Communications)
Meng Yun, the 10th People's Hospital affiliated to Tongji University, Chen Lijie, the postdoctoral fellow of the Department of Materials Science of Fudan University, and Chen Yang, the assistant professor of the School of Life Science and Technology of Tongji University, are the co-first authors of the paper. Bu Wenbo, a professor of the Department of Materials Science of Fudan University, and Wu Yelin, a researcher of the 10th People's Hospital affiliated to Tongji University, and the School of Medicine of Tongji University, are the co-corresponding authors [1].
Two "similarities and differences" between active metal borides and bacteria
Looking back, the topic comes from their finding that the main reason why infected wounds are difficult to heal is that they can not take into account the killing of live bacteria and the inhibition of excessive inflammation induced by dead bacteria.
In order to solve this bottleneck problem, they focused on the key polysaccharide components (lipopolysaccharide/peptidoglycan) shared by living and dead bacteria, drew lessons from the classic esterification reaction between boron dihydroxy and ortho dihydroxy, skillfully utilized the lipopolysaccharide/peptidoglycan of boron dihydroxy complex bacteria produced by the hydrolysis of boric acid materials to block the biological effects of bacteria.
At the same time, in order to overcome the bottleneck problem that the borate ester bond is easy to dissociate under acidic and inflammatory conditions, the team designed and synthesized a new type of active metal boride material system. It is speculated that the hydroxide released by hydrolysis can create an alkaline microenvironment, which helps to improve the stability of the borate ester bond.
Next, the research enters the feasibility verification stage. Specifically, the team adopted the improved high-temperature self-propagating combustion method to prepare a series of active metal borides with particle size of about 200 nm.
Taking magnesium borate as an example, the research team first verified the characteristics of the material to create an alkaline microenvironment by releasing hydroxide during hydrolysis.
During this period, they observed that the active metal borides can form borate ester bonds by complexing with lipopolysaccharide/peptidoglycan. In addition, the preliminary antibacterial experiment proves that magnesium borate has excellent antibacterial properties, which also validates the feasibility of the project.
Subsequently, the research entered the stage of systematically testing the functional properties of materials. The team analyzed the characteristics of the hydrolysis of a series of active metal borides to produce boron dihydroxy, hydroxide and release metal ions, and used infrared spectroscopy and other test technologies to prove that this series of active metal borides can react with lipopolysaccharide/peptidoglycan and bacteria, thus forming borate ester bonds.
Furthermore, through theoretical calculation, they found that the active metal borides can complexe with lipopolysaccharide to form borate ester bonds, and the bond energy is also stronger than the corresponding boric acid complexation. Through SEM-mapping, the team also observed the key experimental data that active metal borides are more easily bound to the bacterial cell wall than boric acid, proving that active metal borides can form stable borate ester bonds with lipopolysaccharide/peptidoglycan.
Next, the research entered the stage of revealing the antibacterial and anti-inflammatory mechanism of active metal borides. During this period, the team has successively used flow cytometry, biological electron microscopy, laser confocal and other test methods to evaluate the excellent antibacterial effect of active metal boride, and observed that active metal boride can change the membrane potential of bacterial cell membrane, while bacterial cell membrane also broke.
Then, through transcriptome sequencing experiments, they found that the cause of bacterial death caused by active metal borides was closely related to the over-activated RNA degradation signal pathway in bacteria
Based on the above results, the team made the following conjecture: the combination of active metal borides and lipopolysaccharide/peptidoglycan on the bacterial surface can significantly increase the local metal cation concentration on the bacterial surface, thus changing the membrane potential of the bacterial outer membrane and damaging the permeability of the membrane, thus activating the bacterial RNA degradation signal pathway, and finally achieving efficient sterilization.
On the other hand, through the immune cell activation experiment, the team also proved that the active metal boride can combine with the free lipopolysaccharide/peptidoglycan released by the dead bacteria, and then inhibit the phosphorylation of MAPKs induced by free lipopolysaccharide/peptidoglycan, including P38, Erk and JNK signal pathways, thereby playing a highly effective anti-inflammatory effect.
The last step is the functional verification stage of living mice. In the mouse model of bacterial infection, the mouse model of inflammation caused by dead bacteria, and the infected wound model, the team systematically verified the anti-infective, anti-inflammatory, and promotive effects of materials on infected wound, and also verified the antibacterial, anti-inflammatory, and promotive effects of active metal borides on infected wound healing from the level of pathological sections and immunofluorescence. At this point, we finally put a complete end to this topic.
For the research process, Bu Wenbo also told a story: "In the early stage of the experiment, we tried to synthesize a series of active metal borides that can be decomposed in water phase and verify whether the material has the envisaged antibacterial function.
The preliminary experimental results have brought us a great surprise - the series of active metal borides have excellent antibacterial activity. It is worth mentioning that for the same bacteria, different types of active metal borides are not the same; For different kinds of bacteria, the same type of active metal borides also show different antibacterial activities. "
This shows that the antibacterial functions of different active metal borides are different, and there is still much room to explore the characteristics of various materials, explore new antibacterial mechanisms of materials, and optimize the antibacterial functions of materials in the future.
"The arsenic of the other, the sweet taste of this"
When designing materials, Bu Wenbo inadvertently communicated with a researcher in the field of superconductivity, and learned that magnesium diboride, a classical material in this field, has a unique phenomenon that decomposes when it meets with moisture and affects the subsequent experimental performance.
"From the thinking of reverse thinking, we found that when this kind of material is applied to the superconducting field, it has the inherent defect of poor stability, but it can be used by the biomedical field, and then turned into a unique performance advantage." Bu Wenbo said.
At the same time, this achievement was jointly completed by the materials workers of Fudan University and the biomedical researchers of the 10th People's Hospital affiliated to Tongji University, which fully reflects the importance of the intersection of medical and industrial work and the close cooperation of personnel from different disciplines. This cooperation mode also gives play to the respective advantages of universities and hospitals.
Next, the team will deeply study the unique "boron capture" material mechanism of active metal borides. As mentioned earlier, in this study, they clearly observed the "boron capture" performance difference between the active metal boride and the common boric acid component. Therefore, they will explore from the perspective of the multi-center bonding characteristics of the boric acid component.
Secondly, the team will also explore the new antibacterial mechanism of active metal boride "boron capture". As mentioned earlier, the antibacterial effect of different active metal borides on the same bacteria is not the same; For different bacteria, even the same active metal boride will present different activities.
Therefore, they will explore relevant molecular biological mechanisms, help screen out antibacterial agents with the best functions, and find the best plan for manufacturing antibacterial agents.
Of course, the team will also strive to promote the clinical conversion of active metal borides. At present, the achievement has been applied for relevant patents. Next, they will systematically study the safety of active metal borides in vivo and in vitro, and develop relevant hydrogels, powders, patches and other products, in order to evaluate the biological activity and stability of such products and ultimately promote clinical transformation.
reference material:
1.Meng, Y., Chen, L., Chen, Y. et al. Reactive metal boride nanoparticles trap lipopolysaccharide and peptidoglycan for bacteria-infected wound healing. Nat Commun 13, 7353 (2022). https://doi.org/10.1038/s41467-022-35050-6
Technological innovation
Honesty is the foundation
Contact Number: +86-15698999555 |
Address: NO.6 ,SHENGHUA STREET,TAIHE DISTRICT, JINZHOU CITY, LIAONING PROVINCE, CHINA. |